Vitamin A
Vitamin A is a group of unsaturated nutritional organic compounds that includes retinol, retinal, retinoic acid, and several provitamin A carotenoids (most notably beta-carotene).[1][2] Vitamin A has multiple functions: it is important for growth and development, for the maintenance of the immune system, and for good vision.[3][4] Vitamin A is needed by the retina of the eye in the form of retinal, which combines with protein opsin to form rhodopsin, the light-absorbing molecule[5] necessary for both low-light (scotopic vision) and color vision.[6] Vitamin A also functions in a very different role as retinoic acid (an irreversibly oxidized form of retinol), which is an important hormone-like growth factor for epithelial and other cells.[4][7]
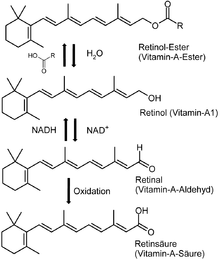
In foods of animal origin, the major form of vitamin A is an ester, primarily retinyl palmitate, which is converted to retinol (chemically an alcohol) in the small intestine. The retinol form functions as a storage form of the vitamin, and can be converted to and from its visually active aldehyde form, retinal.
All forms of vitamin A have a beta-ionone ring to which an isoprenoid chain is attached, called a retinyl group.[1] Both structural features are essential for vitamin activity.[8] The orange pigment of carrots (beta-carotene) can be represented as two connected retinyl groups, which are used in the body to contribute to vitamin A levels. Alpha-carotene and gamma-carotene also have a single retinyl group, which give them some vitamin activity. None of the other carotenes have vitamin activity. The carotenoid beta-cryptoxanthin possesses an ionone group and has vitamin activity in humans.
Vitamin A can be found in two principal forms in foods:
- Retinol, the form of vitamin A absorbed when eating animal food sources, is a yellow, fat-soluble substance. Since the pure alcohol form is unstable, the vitamin is found in tissues in a form of retinyl ester. It is also commercially produced and administered as esters such as retinyl acetate or palmitate.[9]
- The carotenes alpha-carotene, beta-carotene, gamma-carotene; and the xanthophyll beta-cryptoxanthin (all of which contain beta-ionone rings), but no other carotenoids, function as provitamin A in herbivores and omnivore animals, which possess the enzyme beta-carotene 15,15'-dioxygenase which cleaves beta-carotene in the intestinal mucosa and converts it to retinol.[10]
Medical use
Deficiency
Vitamin A deficiency is estimated to affect approximately one third of children under the age of five around the world.[11] It is estimated to claim the lives of 670,000 children under five annually.[12] Between 250,000 and 500,000 children in developing countries become blind each year owing to vitamin A deficiency, with the highest prevalence in Africa and southeast Asia.[13] Vitamin A deficiency is "the leading cause of preventable childhood blindness", according to UNICEF.[14][15] It also increases the risk of death from common childhood conditions such as diarrhea. UNICEF regards addressing vitamin A deficiency as critical to reducing child mortality, the fourth of the United Nations' Millennium Development Goals.[14]
Vitamin A deficiency can occur as either a primary or a secondary deficiency. A primary vitamin A deficiency occurs among children and adults who do not consume an adequate intake of provitamin A carotenoids from fruits and vegetables or preformed vitamin A from animal and dairy products. Early weaning from breastmilk can also increase the risk of vitamin A deficiency.
Secondary vitamin A deficiency is associated with chronic malabsorption of lipids, impaired bile production and release, and chronic exposure to oxidants, such as cigarette smoke, and chronic alcoholism. Vitamin A is a fat-soluble vitamin and depends on micellar solubilization for dispersion into the small intestine, which results in poor use of vitamin A from low-fat diets. Zinc deficiency can also impair absorption, transport, and metabolism of vitamin A because it is essential for the synthesis of the vitamin A transport proteins and as the cofactor in conversion of retinol to retinal. In malnourished populations, common low intakes of vitamin A and zinc increase the severity of vitamin A deficiency and lead physiological signs and symptoms of deficiency.[16] A study in Burkina Faso showed major reduction of malaria morbidity by use of combined vitamin A and zinc supplementation in young children.[17]
Due to the unique function of retinal as a visual chromophore, one of the earliest and specific manifestations of vitamin A deficiency is impaired vision, particularly in reduced light – night blindness. Persistent deficiency gives rise to a series of changes, the most devastating of which occur in the eyes. Some other ocular changes are referred to as xerophthalmia. First there is dryness of the conjunctiva (xerosis) as the normal lacrimal and mucus-secreting epithelium is replaced by a keratinized epithelium. This is followed by the build-up of keratin debris in small opaque plaques (Bitot's spots) and, eventually, erosion of the roughened corneal surface with softening and destruction of the cornea (keratomalacia) and leading to total blindness.[18] Other changes include impaired immunity (increased risk of ear infections, urinary tract infections, meningococcal disease), hyperkeratosis (white lumps at hair follicles), keratosis pilaris and squamous metaplasia of the epithelium lining the upper respiratory passages and urinary bladder to a keratinized epithelium. In relation to dentistry, a deficiency in vitamin A may lead to enamel hypoplasia.
Adequate supply, but not excess vitamin A, is especially important for pregnant and breastfeeding women for normal fetal development and in breastmilk. Deficiencies cannot be compensated by postnatal supplementation.[19][20] Excess vitamin A, which is most common with high-dose vitamin supplements, can cause birth defects and therefore should not exceed recommended daily values.[21]
Vitamin A metabolic inhibition as a result of alcohol consumption during pregnancy is one proposed mechanism for fetal alcohol syndrome, and is characterized by teratogenicity resembling maternal vitamin A deficiency or reduced retinoic acid synthesis during embryogenesis.[22][23][24]
Vitamin A supplementation
A 2012 review found no evidence that beta-carotene or vitamin A supplements increase longevity in healthy people or in people with various diseases.[25] A 2011 review found that vitamin A supplementation of children at risk of deficiency aged under five reduced mortality by up to 24%.[26] However, a 2016 and 2017 Cochrane review concluded there was not evidence to recommend blanket vitamin A supplementation for all infants less than a year of age, as it did not reduce infant mortality or morbidity in low- and middle-income countries.[27][28] The World Health Organization estimated that vitamin A supplementation averted 1.25 million deaths due to vitamin A deficiency in 40 countries since 1998.[29]
While strategies include intake of vitamin A through a combination of breast feeding and dietary intake, delivery of oral high-dose supplements remain the principal strategy for minimizing deficiency.[30] About 75% of the vitamin A required for supplementation activity by developing countries is supplied by the Micronutrient Initiative with support from the Canadian International Development Agency.[31] Food fortification approaches are feasible,[32] but cannot ensure adequate intake levels.[30] Observational studies of pregnant women in sub-Saharan Africa have shown that low serum vitamin A levels are associated with an increased risk of mother-to-child transmission of HIV. Low blood vitamin A levels have been associated with rapid HIV infection and deaths.[33][34] Reviews on the possible mechanisms of HIV transmission found no relationship between blood vitamin A levels in the mother and infant, with conventional intervention established by treatment with anti-HIV drugs.[35][36]
Side effects
Given that vitamin A is fat-soluble, disposing of any excess taken in through diet takes much longer than with water-soluble B vitamins and vitamin C. This allows for toxic levels of vitamin A to accumulate. These toxicities only occur with preformed (retinoid) vitamin A (such as from liver). The carotenoid forms (for example, beta-carotene as found in carrots) give no such symptoms, but excessive dietary intake of beta-carotene can lead to carotenodermia, a harmless but cosmetically displeasing orange-yellow discoloration of the skin.[37][38][39]
In general, acute toxicity occurs at doses of 25,000 IU/kg of body weight, with chronic toxicity occurring at 4,000 IU/kg of body weight daily for 6–15 months.[40] However, liver toxicities can occur at levels as low as 15,000 IU (4500 micrograms) per day to 1.4 million IU per day, with an average daily toxic dose of 120,000 IU, particularly with excessive consumption of alcohol. In people with renal failure, 4000 IU can cause substantial damage. Signs of toxicity may occur with long-term consumption of vitamin A at doses of 25,000–33,000 IU per day.[1]
Excessive vitamin A consumption can lead to nausea, irritability, anorexia (reduced appetite), vomiting, blurry vision, headaches, hair loss, muscle and abdominal pain and weakness, drowsiness, and altered mental status. In chronic cases, hair loss, dry skin, drying of the mucous membranes, fever, insomnia, fatigue, weight loss, bone fractures, anemia, and diarrhea can all be evident on top of the symptoms associated with less serious toxicity.[41] Some of these symptoms are also common to acne treatment with Isotretinoin. Chronically high doses of vitamin A, and also pharmaceutical retinoids such as 13-cis retinoic acid, can produce the syndrome of pseudotumor cerebri.[42] This syndrome includes headache, blurring of vision and confusion, associated with increased intracerebral pressure. Symptoms begin to resolve when intake of the offending substance is stopped.[43]
Chronic intake of 1500 RAE of preformed vitamin A may be associated with osteoporosis and hip fractures because it suppresses bone building while simultaneously stimulating bone breakdown,[44] although other reviews have disputed this effect, indicating further evidence is needed.[1]
A 2012 systematic review found that beta-carotene and higher doses of supplemental vitamin A increased mortality in healthy people and people with various diseases.[25] The findings of the review extend evidence that antioxidants may not have long-term benefits.
Equivalencies of retinoids and carotenoids (IU)
As some carotenoids can be converted into vitamin A, attempts have been made to determine how much of them in the diet is equivalent to a particular amount of retinol, so that comparisons can be made of the benefit of different foods. The situation can be confusing because the accepted equivalences have changed. For many years, a system of equivalencies in which an international unit (IU) was equal to 0.3 μg of retinol, 0.6 μg of β-carotene, or 1.2 μg of other provitamin-A carotenoids was used.[45] Later, a unit called retinol equivalent (RE) was introduced. Prior to 2001, one RE corresponded to 1 μg retinol, 2 μg β-carotene dissolved in oil (it is only partly dissolved in most supplement pills, due to very poor solubility in any medium), 6 μg β-carotene in normal food (because it is not absorbed as well as when in oils), and 12 μg of either α-carotene, γ-carotene, or β-cryptoxanthin in food.
Newer research has shown that the absorption of provitamin-A carotenoids is only half as much as previously thought. As a result, in 2001 the US Institute of Medicine recommended a new unit, the retinol activity equivalent (RAE). Each μg RAE corresponds to 1 μg retinol, 2 μg of β-carotene in oil, 12 μg of "dietary" beta-carotene, or 24 μg of the three other dietary provitamin-A carotenoids.[46]
| Substance and its chemical environment | Proportion of retinol equivalent to substance (μg/μg) |
|---|---|
| Retinol | 1 |
| beta-Carotene, dissolved in oil | 1/2 |
| beta-Carotene, common dietary | 1/12 |
| alpha-Carotene, common dietary | 1/24 |
| gamma-Carotene, common dietary | 1/24 |
| beta-Cryptoxanthin, common dietary | 1/24 |
Because the conversion of retinol from provitamin carotenoids by the human body is actively regulated by the amount of retinol available to the body, the conversions apply strictly only for vitamin A-deficient humans. The absorption of provitamins depends greatly on the amount of lipids ingested with the provitamin; lipids increase the uptake of the provitamin.[47]
A sample vegan diet for one day that provides sufficient vitamin A has been published by the Food and Nutrition Board (page 120[46]). Reference values for retinol or its equivalents, provided by the National Academy of Sciences, have decreased. The RDA (for men) established in 1968 was 5000 IU (1500 μg retinol). In 1974, the RDA was revised to 1000 RE (1000 μg retinol). As of 2001, the RDA for adult males is 900 RAE (900 μg or 3000 IU retinol). By RAE definitions, this is equivalent to 1800 μg of β-carotene supplement dissolved in oil (3000 IU) or 10800 μg of β-carotene in food (18000 IU).
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for vitamin A in 2001. For infants up to 12 months there was not sufficient information to establish a RDA, so Adequate Intake (AI) shown instead. As for safety the IOM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs). The calculation of retinol activity equivalents (RAE) is each μg RAE corresponds to 1 μg retinol, 2 μg of β-carotene in oil, 12 μg of "dietary" beta-carotene, or 24 μg of the three other dietary provitamin-A carotenoids.[46]
| Life stage group |
U.S. RDAs or Adequate Intakes, AI, retinol activity equivalents (μg/day) |
Upper limits, UL* (μg/day) | |
|---|---|---|---|
| Infants | 0–6 months | 400 (AI) | 500 (AI) |
| 7–12 months | 600 | 600 | |
| Children | 1–3 years | 300 | 600 |
| 4–8 years | 400 | 900 | |
| Males | 9–13 years | 600 | 1700 |
| 14–18 years | 900 | 2800 | |
| >19 years | 900 | 3000 | |
| Females | 9–13 years | 600 | 1700 |
| 14–18 years | 700 | 2800 | |
| >19 years | 700 | 3000 | |
| Pregnancy | <19 years | 750 | 2800 |
| >19 years | 770 | 3000 | |
| Lactation | <19 years | 1200 | 2800 |
| >19 years | 1300 | 3000 | |
- ULs are for natural and synthetic retinol ester forms of vitamin A. Beta-carotene and other provitamin A carotenoids from foods and dietary supplements are not added when calculating total vitamin A intake for safety assessments, although they are included as RAEs for RDA and AI calculations.[1][46]
For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For vitamin A labeling purposes 100% of the Daily Value was set at 5,000 IU, but it was revised to 900 μg RAE on May 27, 2016.[48] A table of the pre- and post- adult Daily Values is provided at Reference Daily Intake. The deadline to be in compliance was set for large companies at January 1, 2020, and for small companies at January 1, 2021.[49]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men of ages 15 and older, the PRIs are set respectively at 650 and 750 μg/day. PRI for pregnancy is 700 μg/day, for lactation 1300/day. For children of ages 1–14 years, the PRIs increase with age from 250 to 600 μg/day. These PRIs are similar to the U.S. RDAs.[50] The EFSA reviewed the same safety question as the United States, and set a UL at 3000 μg/day.[51]
Sources
Vitamin A is found in many foods, including the following list.[52] Bracketed values are retinol activity equivalences (RAEs) and percentage of the adult male RDA, per 100 grams of the foodstuff (average). Conversion of carotene to retinol varies from person to person, and bioavailability of carotene in food varies.[53][54]
| Source | Retinol activity equivalences (RAEs) (in μg) | Percentage of the adult male RDA per 100 g of the foodstuff |
|---|---|---|
| cod liver oil | 30000 | 3333% |
| liver turkey | 8058 | 895% |
| liver beef, pork, fish | 6500 | 722% |
| liver chicken | 3296 | 366% |
| ghee | 3069 | 344% |
| sweet potato | 961 | 107% |
| carrot | 835 | 93% |
| broccoli leaf | 800 | 89% |
| butter | 684 | 76% |
| kale | 681 | 76% |
| collard greens frozen then boiled | 575 | 64% |
| butternut squash | 532 | 67% |
| dandelion greens | 508 | 56% |
| spinach | 469 | 52% |
| pumpkin | 426 | 43% |
| collard greens | 333 | 37% |
| cheddar cheese | 265 | 29% |
| cantaloupe melon | 169 | 19% |
| bell pepper/capsicum, red | 157 | 17% |
| egg | 140 | 16% |
| apricot | 96 | 11% |
| papaya | 55 | 6% |
| tomatoes | 42 | 5% |
| mango | 38 | 4% |
| pea | 38 | 4% |
| broccoli florets | 31 | 3% |
| milk | 28 | 3% |
| bell pepper/capsicum, green | 18 | 2% |
| spirulina | 3 | 0.3% |
Metabolic functions
Vitamin A plays a role in a variety of functions throughout the body,[3] such as:
- Vision
- Gene transcription
- Immune function
- Embryonic development and reproduction
- Bone metabolism
- Haematopoiesis
- Skin and cellular health
- Teeth
- Mucous membrane
Vision
The role of vitamin A in the visual cycle is specifically related to the retinal form. Within the eye, 11-cis-retinal is bound to the protein "opsin" to form rhodopsin in rods[5] and iodopsin (cones) at conserved lysine residues. As light enters the eye, the 11-cis-retinal is isomerized to the all-"trans" form. The all-"trans" retinal dissociates from the opsin in a series of steps called photo-bleaching. This isomerization induces a nervous signal along the optic nerve to the visual center of the brain. After separating from opsin, the all-"trans"-retinal is recycled and converted back to the 11-"cis"-retinal form by a series of enzymatic reactions. In addition, some of the all-"trans" retinal may be converted to all-"trans" retinol form and then transported with an interphotoreceptor retinol-binding protein (IRBP) to the pigment epithelial cells. Further esterification into all-"trans" retinyl esters allow for storage of all-trans-retinol within the pigment epithelial cells to be reused when needed.[16] The final stage is conversion of 11-cis-retinal will rebind to opsin to reform rhodopsin (visual purple) in the retina. Rhodopsin is needed to see in low light (contrast) as well as for night vision. Kühne showed that rhodopsin in the retina is only regenerated when the retina is attached to retinal pigmented epithelium,[5] which provides retinal. It is for this reason that a deficiency in vitamin A will inhibit the reformation of rhodopsin, and will lead to one of the first symptoms, night blindness.[55]
Gene transcription
Vitamin A, in the retinoic acid form, plays an important role in gene transcription. Once retinol has been taken up by a cell, it can be oxidized to retinal (retinaldehyde) by retinol dehydrogenases; retinaldehyde can then be oxidized to retinoic acid by retinaldehyde dehydrogenases.[21] The conversion of retinaldehyde to retinoic acid is an irreversible step; this means that the production of retinoic acid is tightly regulated, due to its activity as a ligand for nuclear receptors.[16] The physiological form of retinoic acid (all-trans-retinoic acid) regulates gene transcription by binding to nuclear receptors known as retinoic acid receptors (RARs) which are bound to DNA as heterodimers with retinoid "X" receptors (RXRs). RAR and RXR must dimerize before they can bind to the DNA. RAR will form a heterodimer with RXR (RAR-RXR), but it does not readily form a homodimer (RAR-RAR). RXR, on the other hand, may form a homodimer (RXR-RXR) and will form heterodimers with many other nuclear receptors as well, including the thyroid hormone receptor (RXR-TR), the Vitamin D3 receptor (RXR-VDR), the peroxisome proliferator-activated receptor (RXR-PPAR) and the liver "X" receptor (RXR-LXR).[56]
The RAR-RXR heterodimer recognizes retinoic acid response elements (RAREs) on the DNA whereas the RXR-RXR homodimer recognizes retinoid "X" response elements (RXREs) on the DNA; although several RAREs near target genes have been shown to control physiological processes,[21] this has not been demonstrated for RXREs. The heterodimers of RXR with nuclear receptors other than RAR (i.e. TR, VDR, PPAR, LXR) bind to various distinct response elements on the DNA to control processes not regulated by vitamin A.[16] Upon binding of retinoic acid to the RAR component of the RAR-RXR heterodimer, the receptors undergo a conformational change that causes co-repressors to dissociate from the receptors. Coactivators can then bind to the receptor complex, which may help to loosen the chromatin structure from the histones or may interact with the transcriptional machinery.[56] This response can upregulate (or downregulate) the expression of target genes, including Hox genes as well as the genes that encode for the receptors themselves (i.e. RAR-beta in mammals).[16]
Immune function
Vitamin A plays a role in many areas of the immune system, particularly in T cell differentiation and proliferation.[57][58]
Vitamin A promotes the proliferation of T cells through an indirect mechanism involving an increase in IL-2.[58] In addition to promoting proliferation, vitamin A (specifically retinoic acid) influences the differentiation of T cells.[59][60] In the presence of retinoic acid, dendritic cells located in the gut are able to mediate the differentiation of T cells into regulatory T cells.[60] Regulatory T cells are important for prevention of an immune response against "self" and regulating the strength of the immune response in order to prevent host damage. Together with TGF-β, Vitamin A promotes the conversion of T cells to regulatory T cells.[59] Without Vitamin A, TGF-β stimulates differentiation into T cells that could create an autoimmune response.[59]
Hematopoietic stem cells are important for the production of all blood cells, including immune cells, and are able to replenish these cells throughout the life of an individual. Dormant hematopoietic stem cells are able to self-renew, and are available to differentiate and produce new blood cells when they are needed. In addition to T cells, Vitamin A is important for the correct regulation of hematopoietic stem cell dormancy.[61] When cells are treated with all-trans retinoic acid, they are unable to leave the dormant state and become active, however, when vitamin A is removed from the diet, hematopoietic stem cells are no longer able to become dormant and the population of hematopoietic stem cells decreases.[61] This shows an importance in creating a balanced amount of vitamin A within the environment to allow these stem cells to transition between a dormant and activated state, in order to maintain a healthy immune system.
Vitamin A has also been shown to be important for T cell homing to the intestine, effects dendritic cells, and can play a role in increased IgA secretion, which is important for the immune response in mucosal tissues.[57][62]
Dermatology
Vitamin A, and more specifically, retinoic acid, appears to maintain normal skin health by switching on genes and differentiating keratinocytes (immature skin cells) into mature epidermal cells.[63] Exact mechanisms behind pharmacological retinoid therapy agents in the treatment of dermatological diseases are being researched. For the treatment of acne, the most prescribed retinoid drug is 13-cis retinoic acid (isotretinoin). It reduces the size and secretion of the sebaceous glands. Although it is known that 40 mg of isotretinoin will break down to an equivalent of 10 mg of ATRA — the mechanism of action of the drug (original brand name Accutane) remains unknown and is a matter of some controversy. Isotretinoin reduces bacterial numbers in both the ducts and skin surface. This is thought to be a result of the reduction in sebum, a nutrient source for the bacteria. Isotretinoin reduces inflammation via inhibition of chemotactic responses of monocytes and neutrophils.[16] Isotretinoin also has been shown to initiate remodeling of the sebaceous glands; triggering changes in gene expression that selectively induce apoptosis.[64] Isotretinoin is a teratogen with a number of potential side-effects. Consequently, its use requires medical supervision.
Retinal/retinol versus retinoic acid
Vitamin A-deprived rats can be kept in good general health with supplementation of retinoic acid. This reverses the growth-stunting effects of vitamin A deficiency, as well as early stages of xerophthalmia. However, such rats show infertility (in both male and females) and continued degeneration of the retina, showing that these functions require retinal or retinol, which are interconvertible but which cannot be recovered from the oxidized retinoic acid. The requirement of retinol to rescue reproduction in vitamin A deficient rats is now known to be due to a requirement for local synthesis of retinoic acid from retinol in testis and embryos.[65][66]
Vitamin A and derivatives in medical use
Retinyl palmitate has been used in skin creams, where it is broken down to retinol and ostensibly metabolised to retinoic acid, which has potent biological activity, as described above. The retinoids (for example, 13-cis-retinoic acid) constitute a class of chemical compounds chemically related to retinoic acid, and are used in medicine to modulate gene functions in place of this compound. Like retinoic acid, the related compounds do not have full vitamin A activity, but do have powerful effects on gene expression and epithelial cell differentiation.[67] Pharmaceutics utilizing megadoses of naturally occurring retinoic acid derivatives are currently in use for cancer, HIV, and dermatological purposes.[68] At high doses, side-effects are similar to vitamin A toxicity.
History
The discovery of vitamin A may have stemmed from research dating back to 1816, when physiologist François Magendie observed that dogs deprived of nutrition developed corneal ulcers and had a high mortality rate.[69] In 1912, Frederick Gowland Hopkins demonstrated that unknown accessory factors found in milk, other than carbohydrates, proteins, and fats were necessary for growth in rats. Hopkins received a Nobel Prize for this discovery in 1929.[69][70] By 1913, one of these substances was independently discovered by Elmer McCollum and Marguerite Davis at the University of Wisconsin–Madison, and Lafayette Mendel and Thomas Burr Osborne at Yale University, who studied the role of fats in the diet. McCollum and Davis ultimately received credit because they submitted their paper three weeks before Mendel and Osborne. Both papers appeared in the same issue of the Journal of Biological Chemistry in 1913.[71] The "accessory factors" were termed "fat soluble" in 1918 and later "vitamin A" in 1920. In 1919, Harry Steenbock (University of Wisconsin–Madison) proposed a relationship between yellow plant pigments (beta-carotene) and vitamin A. In 1931, Swiss chemist Paul Karrer described the chemical structure of vitamin A.[69] Vitamin A was first synthesized in 1947 by two Dutch chemists, David Adriaan van Dorp and Jozef Ferdinand Arens.
During World War II, German bombers would attack at night to evade British defenses. In order to keep the 1939 invention of a new on-board Airborne Intercept Radar system secret from German bombers, the British Royal Ministry told newspapers that the nighttime defensive success of Royal Air Force pilots was due to a high dietary intake of carrots rich in vitamin A, propagating the myth that carrots enable people to see better in the dark.[72]
References
- "Vitamin A". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. January 2015. Retrieved 6 July 2017.
- Fennema O (2008). Fennema's Food Chemistry. CRC Press/Taylor & Francis. pp. 454–455. ISBN 9780849392726.
- "Vitamin A". MedlinePlus, National Library of Medicine, US National Institutes of Health. 2 December 2016.
- Tanumihardjo SA (August 2011). "Vitamin A: biomarkers of nutrition for development". The American Journal of Clinical Nutrition. 94 (2): 658S–65S. doi:10.3945/ajcn.110.005777. PMC 3142734. PMID 21715511.
- Wolf G (June 2001). "The discovery of the visual function of vitamin A". The Journal of Nutrition. 131 (6): 1647–50. doi:10.1093/jn/131.6.1647. PMID 11385047.
- "Vitamin A". Office of Dietary Supplements, US National Institutes of Health. 31 August 2016.
- News Medical. "What is Vitamin A?". Retrieved 1 May 2012.
- Berdanier C (1997). Advanced Nutrition Micronutrients. CRC Press. pp. 22–39. ISBN 978-0-8493-2664-6.
- Meschino Health. "Comprehensive Guide to Vitamin A". Archived from the original on 15 May 2013. Retrieved 1 May 2012.
- DeMan J (1999). Principles of Food chemistry (3rd ed.). Maryland: Aspen Publication Inc. p. 358. ISBN 978-0834212343.
- "Global prevalence of vitamin A deficiency in populations at risk 1995–2005" (PDF). WHO global database on vitamin A deficiency. World Health Organization. 2009.
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J (January 2008). "Maternal and child undernutrition: global and regional exposures and health consequences". The Lancet. 371 (9608): 243–60. doi:10.1016/S0140-6736(07)61690-0. PMID 18207566.
- "Fact sheet for health professionals: Vitamin A". Office of Dietary Supplements, National Institutes of Health. 5 June 2013. Retrieved 6 December 2015.
- "Vitamin A Deficiency", UNICEF. Retrieved 3 June 2015.
- Also see Akhtar S, Ahmed A, Randhawa MA, Atukorala S, Arlappa N, Ismail T, Ali Z (December 2013). "Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies". Journal of Health, Population, and Nutrition. 31 (4): 413–23. doi:10.3329/jhpn.v31i4.19975. PMC 3905635. PMID 24592582.
- Combs GF (2008). The Vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Burlington, MA: Elsevier Academic Press. ISBN 978-0-12-183493-7.
- Zeba AN, Sorgho H, Rouamba N, Zongo I, Rouamba J, Guiguemdé RT, Hamer DH, Mokhtar N, Ouedraogo JB (January 2008). "Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial". Nutrition Journal. 7: 7. doi:10.1186/1475-2891-7-7. PMC 2254644. PMID 18237394.
- Roncone DP (March 2006). "Xerophthalmia secondary to alcohol-induced malnutrition". Optometry. 77 (3): 124–33. doi:10.1016/j.optm.2006.01.005. PMID 16513513.
- Strobel M, Tinz J, Biesalski HK (July 2007). "The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women". European Journal of Nutrition. 46 Suppl 1: I1–20. doi:10.1007/s00394-007-1001-z. PMID 17665093.
- Schulz C, Engel U, Kreienberg R, Biesalski HK (February 2007). "Vitamin A and beta-carotene supply of women with gemini or short birth intervals: a pilot study". European Journal of Nutrition. 46 (1): 12–20. doi:10.1007/s00394-006-0624-9. PMID 17103079.
- Duester G (September 2008). "Retinoic acid synthesis and signaling during early organogenesis". Cell. 134 (6): 921–31. doi:10.1016/j.cell.2008.09.002. PMC 2632951. PMID 18805086.
- Deltour L, Ang HL, Duester G (July 1996). "Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome". FASEB Journal. 10 (9): 1050–7. doi:10.1096/fasebj.10.9.8801166. PMID 8801166.
- Crabb DW, Pinairs J, Hasanadka R, Fang M, Leo MA, Lieber CS, et al. (May 2001). "Alcohol and retinoids". Alcoholism, Clinical and Experimental Research. 25 (5 Suppl ISBRA): 207S–217S. doi:10.1111/j.1530-0277.2001.tb02398.x. PMID 11391073.
- Shabtai Y, Bendelac L, Jubran H, Hirschberg J, Fainsod A (January 2018). "Acetaldehyde inhibits retinoic acid biosynthesis to mediate alcohol teratogenicity". Scientific Reports. 8 (1): 347. doi:10.1038/s41598-017-18719-7. PMC 5762763. PMID 29321611.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (March 2012). "Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases". The Cochrane Database of Systematic Reviews. 3 (3): CD007176. doi:10.1002/14651858.CD007176.pub2. hdl:10138/136201. PMID 22419320.
- Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA (August 2011). "Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis". BMJ. 343: d5094. doi:10.1136/bmj.d5094. PMC 3162042. PMID 21868478.
- Imdad A, Ahmed Z, Bhutta ZA (September 2016). "Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age". The Cochrane Database of Systematic Reviews. 9: CD007480. doi:10.1002/14651858.CD007480.pub3. PMC 6457829. PMID 27681486.
- Haider BA, Sharma R, Bhutta ZA (February 2017). "Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries". The Cochrane Database of Systematic Reviews. 2: CD006980. doi:10.1002/14651858.CD006980.pub3. PMC 6464547. PMID 28234402.
- "Micronutrient Deficiencies-Vitamin A". World Health Organization. Retrieved 9 April 2008.
- Vitamin A Supplementation: A Decade of Progress (PDF). New York: UNICEF. 2007. p. 3. ISBN 978-92-806-4150-9.
- Micronutrient Initiative Annual Report (PDF). 2016–2017. p. 4.
- Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA (June 2009). "Golden Rice is an effective source of vitamin A". The American Journal of Clinical Nutrition. 89 (6): 1776–83. doi:10.3945/ajcn.2008.27119. PMC 2682994. PMID 19369372.
- Semba RD, Caiaffa WT, Graham NM, Cohn S, Vlahov D (May 1995). "Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users". The Journal of Infectious Diseases. 171 (5): 1196–202. doi:10.1093/infdis/171.5.1196. PMID 7751694.
- Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D (September 1993). "Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection". Archives of Internal Medicine. 153 (18): 2149–54. doi:10.1001/archinte.1993.00410180103012. PMID 8379807.
- Wiysonge CS, Ndze VN, Kongnyuy EJ, Shey MS (September 2017). "Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection". The Cochrane Database of Systematic Reviews. 9: CD003648. doi:10.1002/14651858.CD003648.pub4. PMC 5618453. PMID 28880995.
- "Guideline: Vitamin A supplementation in pregnancy for reducing the risk of mother-to-child transmission of HIV" (PDF). World Health Organization. 2011. Retrieved 4 March 2015.
- Sale TA, Stratman E (2004). "Carotenemia associated with green bean ingestion". Pediatric Dermatology. 21 (6): 657–9. doi:10.1111/j.0736-8046.2004.21609.x. PMID 15575851.
- Nishimura Y, Ishii N, Sugita Y, Nakajima H (October 1998). "A case of carotenodermia caused by a diet of the dried seaweed called Nori". The Journal of Dermatology. 25 (10): 685–7. doi:10.1111/j.1346-8138.1998.tb02482.x. PMID 9830271.
- Takita Y, Ichimiya M, Hamamoto Y, Muto M (February 2006). "A case of carotenemia associated with ingestion of nutrient supplements". The Journal of Dermatology. 33 (2): 132–4. doi:10.1111/j.1346-8138.2006.00028.x. PMID 16556283.
- Rosenbloom, Mark. "Toxicity, Vitamin". eMedicine.
- Eledrisi, Mohsen S. "Vitamin A Toxicity". eMedicine.
- Brazis PW (March 2004). "Pseudotumor cerebri". Current Neurology and Neuroscience Reports. 4 (2): 111–6. doi:10.1007/s11910-004-0024-6. PMID 14984682.
- AJ Giannini, RL Gilliland. The Neurologic, Neurogenic and Neuropsychiatric Disorders Handbook. New Hyde Park, NY. Medical Examination Publishing Co., 1982, ISBN 0-87488-699-6 pp. 182–183.
- Whitney E, Rolfes SR (2011). Williams P (ed.). Understanding Nutrition (Twelfth ed.). California: Wadsworth:Cengage Learning. ISBN 978-0-538-73465-3.
- Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 20 USDA, Feb. 2008
- Vitamin A of Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, Food and Nutrition Board of the Institute of Medicine, pages 82–161. 2001
- Solomons NW, Orozco M (2003). "Alleviation of vitamin A deficiency with palm fruit and its products". Asia Pacific Journal of Clinical Nutrition. 12 (3): 373–84. PMID 14506004.
- "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels" (PDF).
- "Changes to the Nutrition Facts Panel - Compliance Date"
- "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- "Rank order of vitamin A content in foods per 100 g". USDA National Nutrient Database. 29 March 2017. Retrieved 26 April 2017.
- Borel P, Drai J, Faure H, Fayol V, Galabert C, Laromiguière M, Le Moël G (2005). "[Recent knowledge about intestinal absorption and cleavage of carotenoids]". Annales de Biologie Clinique (in French). 63 (2): 165–77. PMID 15771974.
- Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA (October 2005). "Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables". The American Journal of Clinical Nutrition. 82 (4): 821–8. doi:10.1093/ajcn/82.4.821. PMID 16210712.
- McGuire M, Beerman KA (2007). Nutritional sciences: from fundamentals to food. Belmont, CA: Thomson/Wadsworth. ISBN 978-0-534-53717-3.
- Stipanuk MH (2006). Biochemical, Physiological and Molecular Aspects of Human Nutrition (2nd ed.). Philadelphia: Saunders. ISBN 9781416002093.
- Mora JR, Iwata M, von Andrian UH (September 2008). "Vitamin effects on the immune system: vitamins A and D take centre stage". Nature Reviews. Immunology. 8 (9): 685–98. doi:10.1038/nri2378. PMC 2906676. PMID 19172691.
- Ertesvag A, Engedal N, Naderi S, Blomhoff HK (November 2002). "Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion". Journal of Immunology. 169 (10): 5555–63. doi:10.4049/jimmunol.169.10.5555. PMID 12421932.
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H (July 2007). "Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid". Science. 317 (5835): 256–60. doi:10.1126/science.1145697. PMID 17569825.
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y (August 2007). "Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid". The Journal of Experimental Medicine. 204 (8): 1775–85. doi:10.1084/jem.20070602. PMC 2118682. PMID 17620362.
- Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, Pastor-Flores D, Roma LP, Renders S, Zeisberger P, Przybylla A, Schönberger K, Scognamiglio R, Altamura S, Florian CM, Fawaz M, Vonficht D, Tesio M, Collier P, Pavlinic D, Geiger H, Schroeder T, Benes V, Dick TP, Rieger MA, Stegle O, Trumpp A (May 2017). "Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy". Cell. 169 (5): 807–823.e19. doi:10.1016/j.cell.2017.04.018. PMID 28479188.
- Ross AC (November 2012). "Vitamin A and retinoic acid in T cell-related immunity". The American Journal of Clinical Nutrition. 96 (5): 1166S–72S. doi:10.3945/ajcn.112.034637. PMC 3471201. PMID 23053562.
- Fuchs E, Green H (September 1981). "Regulation of terminal differentiation of cultured human keratinocytes by vitamin A". Cell. 25 (3): 617–25. doi:10.1016/0092-8674(81)90169-0. PMID 6169442.
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM (April 2008). "Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells". The Journal of Clinical Investigation. 118 (4): 1468–78. doi:10.1172/JCI33869. PMC 2262030. PMID 18317594.
- Moore T, Holmes PD (October 1971). "The production of experimental vitamin A deficiency in rats and mice". Laboratory Animals. 5 (2): 239–50. doi:10.1258/002367771781006492. PMID 5126333.
- van Beek ME, Meistrich ML (March 1992). "Spermatogenesis in retinol-deficient rats maintained on retinoic acid". Journal of Reproduction and Fertility. 94 (2): 327–36. doi:10.1530/jrf.0.0940327. PMID 1593535.
- American Cancer Society: Retinoid Therapy
- Vivat-Hannah V, Zusi FC (August 2005). "Retinoids as therapeutic agents: today and tomorrow". Mini Reviews in Medicinal Chemistry. 5 (8): 755–60. doi:10.2174/1389557054553820. PMID 16101411.
- Semba RD (2012). "On the 'discovery' of vitamin A". Annals of Nutrition & Metabolism. 61 (3): 192–8. doi:10.1159/000343124. PMID 23183288.
- Wolf G (2001). "Discovery of Vitamin A". Encyclopedia of Life Sciences. doi:10.1038/npg.els.0003419. ISBN 978-0-470-01617-6.
- Rosenfeld L (April 1997). "Vitamine—vitamin. The early years of discovery". Clinical Chemistry. American Association for Clinical Chemistry. 43 (4): 680–5. PMID 9105273.
- K. Annabelle Smith (13 August 2013). "A WWII Propaganda Campaign Popularized the Myth That Carrots Help You See in the Dark". Smithsonian.com. Retrieved 2 May 2018.
Further reading
- Litwack G (2007). Vitamin A. Vitamins and Hormones. 75. San Diego, CA: Elsevier Academic Press. ISBN 978-0-12-709875-3.
- "Vitamin A Supplementation: A Decade of Progress" (PDF). New York: UNICEF. 2007.
- "Investing in the Future: A United Call to Action on Vitamin and Mineral Deficiencies" (PDF). GAIN, Micronutrient Initiative, USAID, The World Bank, UNICEF, Flour Fortification Initiative. 2009.
External links
- Vitamin+A at the US National Library of Medicine Medical Subject Headings (MeSH)
- World Health Organization Database on Vitamin A Deficiency