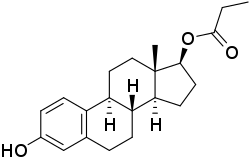
Estradiol monopropionate
Estradiol propionate (EP), also known as estradiol monopropionate or estradiol 17β-propionate and sold under the brand names Acrofollin, Akrofollin, and Follhormon, is an estrogen medication and estrogen ester which is no longer marketed.[1][2] It is the C17β propionate ester of estradiol.[1][2] EP was provided in an oil solution and was administered by intramuscular injection.[3][4][5][6] The medication was first marketed by 1938 or 1939.[7][8]
 | |
| Clinical data | |
|---|---|
| Trade names | Acrofollin, Akrofollin, Follhormon |
| Other names | EP; Estradiol monopropionate; Estradiol propanoate; Estradiol 17β-propionate; Estradiol 17β-propanoate; Estra-1,3,5(10)-trien-3,17β-diol 17β-propionate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H28O3 |
| Molar mass | 328.445 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 898. ISBN 978-1-4757-2085-3.
- Martin Negwer (1994). Organic-chemical Drugs and Their Synonyms: (an International Survey). Akademie Verlag. p. 1967. ISBN 978-3-05-500156-7.
Estra-1,3,5(10)-triene-3,17β-diol 17-propionate = 3,17β-Estradiol 17-monopropionate = (17β)-Estra-1,3,5(10)-triene-3,17-diol 17-propanoate (e) S Acrofollin, Akrofollin, Estradiol monopropionate, Follhormon "Saper", Ostradiolmonopropionat, Oestrolum propionicum U Estrogen
- United States. Department of the Treasury (1942). Treasury Decisions Under the Customs, Internal Revenue, and Other Laws: Including the Decisions of the Board of General Appraisers and the Court of Customs Appeals. U.S. Government Printing Office. p. 135.
[...] T. D. 50714-C, covering akrofollin intramuscular oily solution of estrogenic hormone manufactured by Specific Pharmaceuticals, Inc., New York, N. Y., with the use of imported oestradiol-17-propionate crystals, [...]
- Csillag, M.; Vaczy, L.; Türr, E. (1951). "Apparent Differences between the Parenteral and Intrauterine Administration of Estrogen Substances". Gynecologic and Obstetric Investigation. 131 (1): 9–18. doi:10.1159/000311707. ISSN 1423-002X.
For the following two groups we have chosen estradiol propionate (Akrofollin) in a dose of 3 X 5 mg. In each instance we performed biopsic strip-abrasion on the third day after the termination of intrauterine, resp. intramuscular hormone administration.
- Orosz, Mihály; Csapó, István; Varga, Bertalan (1983). "Alteration in the reactivity of hamster cheek pouch arterioles to prostaglandin E2 and noradrenaline during pregnancy or sex steroid treatment". Prostaglandins. 26 (2): 165–173. doi:10.1016/0090-6980(83)90085-0. ISSN 0090-6980.
[...] they were treated each second day with 0.2 ml sunflower oil, 0.2 mg/lOO g bw of oestradiol propionate (Akrofollin, Richter), [...]
- Wachnik, Anna; Biró, G.; Biró, L.; Korom, M.; Gergely, Anna; Antal, Magdolna (1993). "Effect of sex hormones on copper, zinc, iron nutritional status and hepatic lipid peroxidation in rats". Food/Nahrung. 37 (1): 28–34. doi:10.1002/food.19930370106. ISSN 0027-769X.
injected subcutaneously with oily solution of estradiol propionate (Acrofollin, Richter Gedeon)
- Boletín oficial de la propiedad industrial. Carasa y Cía. 1938.
Fecha del Fecha de la solicitud de la marca: Abril 13, 1938. Certificado de propiedad: Octubre 23, 1939. Nombre y domicilio del concesionario: Chinoin Fábrica de Productos Farmacéuticos y Química S. A., Ujpest, Hungría. AKROFOLLIN.
- Dubrauszky, V.; Martzy, St. (1941). "Die Wirkung natürlicher und künstlicher Brunststoffe im Tierversuch" [The effect of natural and artificial compounds in animal experiments]. Archiv für Gynäkologie. 171 (2): 242–253. doi:10.1007/BF01714680. ISSN 0003-9128.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.