White matter
White matter refers to areas of the central nervous system (CNS) that are mainly made up of myelinated axons, also called tracts.[1] Long thought to be passive tissue, white matter affects learning and brain functions, modulating the distribution of action potentials, acting as a relay and coordinating communication between different brain regions.[2]
| White matter | |
|---|---|
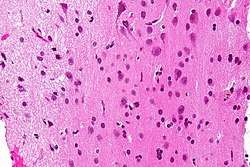 Micrograph showing white matter with its characteristic fine meshwork-like appearance (left of image - lighter shade of pink) and grey matter, with the characteristic neuronal cell bodies (right of image - dark shade of pink). HPS stain. | |
Human brain right dissected lateral view, showing grey matter (the darker outer parts), and white matter (the inner and prominently whiter parts). | |
| Details | |
| Identifiers | |
| Latin | substantia alba |
| MeSH | D066127 |
| TA | A14.1.00.009 |
| FMA | 83929 |
| Anatomical terminology | |
White matter is named for its relatively light appearance resulting from the lipid content of myelin. However, the tissue of the freshly cut brain appears pinkish white to the naked eye because myelin is composed largely of lipid tissue veined with capillaries. Its white color in prepared specimens is due to its usual preservation in formaldehyde.
Structure
White matter
White matter is composed of bundles, which connect various gray matter areas (the locations of nerve cell bodies) of the brain to each other, and carry nerve impulses between neurons. Myelin acts as an insulator, which allows electrical signals to jump, rather than coursing through the axon, increasing the speed of transmission of all nerve signals.[3]
The total number of long range fibers within a cerebral hemisphere is 2% of the total number of cortico-cortical fibers (across cortical areas) and is roughly the same number as those that communicate between the two hemispheres in the brain's largest white tissue structure, the corpus callosum.[4] Schüz and Braitenberg note "As a rough rule, the number of fibres of a certain range of lengths is inversely proportional to their length."[4]
White matter in nonelderly adults is 1.7–3.6% blood.[5]
Grey matter
The other main component of the brain is grey matter (actually pinkish tan due to blood capillaries), which is composed of neurons. The substantia nigra is a third colored component found in the brain that appears darker due to higher levels of melanin in dopaminergic neurons than its nearby areas. Note that white matter can sometimes appear darker than grey matter on a microscope slide because of the type of stain used. Cerebral- and spinal white matter do not contain dendrites, neural cell bodies, or shorter axons, which can only be found in grey matter.
Location
White matter forms the bulk of the deep parts of the brain and the superficial parts of the spinal cord. Aggregates of grey matter such as the basal ganglia (caudate nucleus, putamen, globus pallidus, substantia nigra, subthalamic nucleus, nucleus accumbens) and brainstem nuclei (red nucleus, cranial nerve nuclei) are spread within the cerebral white matter.
The cerebellum is structured in a similar manner as the cerebrum, with a superficial mantle of cerebellar cortex, deep cerebellar white matter (called the "arbor vitae") and aggregates of grey matter surrounded by deep cerebellar white matter (dentate nucleus, globose nucleus, emboliform nucleus, and fastigial nucleus). The fluid-filled cerebral ventricles (lateral ventricles, third ventricle, cerebral aqueduct, fourth ventricle) are also located deep within the cerebral white matter.
Myelinated axon length
Men have more white matter than females both in volume and in length of myelinated axons. At the age of 20, the total length of myelinated fibers in males is 176,000 km while that of a female is 149,000 km.[6] There is a decline in total length with age of about 10% each decade such that a man at 80 years of age has 97,200 km and a female 82,000 km.[6] Most of this reduction is due to the loss of thinner fibers.[6]
Function
White matter is the tissue through which messages pass between different areas of gray matter within the central nervous system. The white matter is white because of the fatty substance (myelin) that surrounds the nerve fibers (axons). This myelin is found in almost all long nerve fibers, and acts as an electrical insulation. This is important because it allows the messages to pass quickly from place to place.
Unlike gray matter, which peaks in development in a person's twenties, the white matter continues to develop, and peaks in middle age.[7]
Clinical significance
Multiple sclerosis (MS) is one of the most common diseases which affect white matter. In MS lesions, the myelin shield around the axons has been destroyed by inflammation.
Alcohol use disorders are associated with decrease in white matter volume.[8] Animal studies suggest that alcohol may cause loss of white matter by damaging oligodendrocytes, the glial cell responsible for maintaining myelin.[9]
Changes in the white matter known as amyloid plaques are associated with Alzheimer's disease and other neurodegenerative diseases. White matter injuries ("axonal shearing") may be reversible, while gray matter regeneration is less likely. Other changes that commonly occur with age include the development of leukoaraiosis, which is a rarefaction of the white matter that can be correlated with a variety of conditions, including loss of myelin pallor, axonal loss, and a breakdown of the blood–brain barrier.[10]
White matter lesions on magnetic resonance imaging are linked to several adverse outcomes, such as cognitive impairment, functional disability, death, neurologic problems, and depression.[11]
White matter hyperintensity are more than often present with vascular dementia, particularly among small vessel/subcortical subtypes of vascular dementia as well as Binswanger’s disease, and are generally thought to be an eventuality of chronic ischemia associated with microangiopathy.[12]
Volume
Smaller volumes (in terms of group averages) of white matters might be associated with larger deficits in attention, declarative memory, executive functions, intelligence, and academic achievement.[13][14][15] However, volumic change is continuous throughout one's lifetime due to neuroplasticity and is a contributing factor rather than determinant factor of certain functional deficits, this is because of assistance owing to compensating effects from other parts of the brain.[15] Starting at one's late adulthood, typically after sixty, the integrity of white matter declines due to aging.[16] Nonetheless, regular aerobic exercise appears to either postpone the aging effect or in turn enhance the white matter integrity in the long run.[16]
White matter size might be interactive with the severity of obstructive sleep apnea in the long run.[17][18][19][20][21]
Imaging
The study of white matter has been advanced with the neuroimaging technique called diffusion tensor imaging where magnetic resonance imaging (MRI) brain scanners are used. As of 2007, more than 700 publications have been published on the subject.[22]
A 2009 paper by Jan Scholz and colleagues[23] used diffusion tensor imaging (DTI) to demonstrate changes in white matter volume as a result of learning a new motor task (e.g. juggling). The study is important as the first paper to correlate motor learning with white matter changes. Previously, many researchers had considered this type of learning to be exclusively mediated by dendrites, which are not present in white matter. The authors suggest that electrical activity in axons may regulate myelination in axons. Or, gross changes in the diameter or packing density of the axon might cause the change.[24] A more recent DTI study by Sampaio-Baptista and colleagues reported changes in white matter with motor learning along with increases in myelination.[25]
See also
References
- Blumenfeld, Hal (2010). Neuroanatomy through clinical cases (2nd ed.). Sunderland, Mass.: Sinauer Associates. p. 21. ISBN 9780878936137.
Areas of the CNS made up mainly of myelinated axons are called white matter.
- Douglas Fields, R. (2008). "White Matter Matters". Scientific American. 298 (3): 54–61. Bibcode:2008SciAm.298c..54D. doi:10.1038/scientificamerican0308-54.
- Klein, S.B., & Thorne, B.M. Biological Psychology. Worth Publishers: New York. 2007.
- Schüz, Almut; Braitenberg, Valentino (2002). "The human cortical white matter: Quantitative aspects of cortico-cortical long-range connectivity". In Schüz, Almut; Braitenberg, Valentino (eds.). Cortical Areas: Unity and Diversity, Conceptual Advances in Brain Research. Taylor and Francis. pp. 377–86. ISBN 978-0-415-27723-5.
- Leenders, K. L.; Perani, D.; Lammertsma, A. A.; Heather, J. D.; Buckingham, P.; Jones, T.; Healy, M. J. R.; Gibbs, J. M.; Wise, R. J. S.; Hatazawa, J.; Herold, S.; Beaney, R. P.; Brooks, D. J.; Spinks, T.; Rhodes, C.; Frackowiak, R. S. J. (1990). "Cerebral Blood Flow, Blood Volume and Oxygen Utilization". Brain. 113: 27–47. doi:10.1093/brain/113.1.27. PMID 2302536.
- Marner, Lisbeth; Nyengaard, Jens R.; Tang, Yong; Pakkenberg, Bente (2003). "Marked loss of myelinated nerve fibers in the human brain with age". The Journal of Comparative Neurology. 462 (2): 144–52. doi:10.1002/cne.10714. PMID 12794739.
- Sowell, Elizabeth R.; Peterson, Bradley S.; Thompson, Paul M.; Welcome, Suzanne E.; Henkenius, Amy L.; Toga, Arthur W. (2003). "Mapping cortical change across the human life span". Nature Neuroscience. 6 (3): 309–15. doi:10.1038/nn1008. PMID 12548289.
- Monnig, Mollie A.; Tonigan, J. Scott; Yeo, Ronald A.; Thoma, Robert J.; McCrady, Barbara S. (2013). "White matter volume in alcohol use disorders: A meta-analysis". Addiction Biology. 18 (3): 581–92. doi:10.1111/j.1369-1600.2012.00441.x. PMC 3390447. PMID 22458455.
- Alfonso-Loeches, Silvia; Pascual, Maria; Gómez-Pinedo, Ulises; Pascual-Lucas, Maya; Renau-Piqueras, Jaime; Guerri, Consuelo (2012). "Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse". Glia. 60 (6): 948–64. doi:10.1002/glia.22327. PMID 22431236.
- O'Sullivan, M. (2008-01-01). "Leukoaraiosis". Practical Neurology. 8 (1): 26–38. doi:10.1136/jnnp.2007.139428. ISSN 1474-7758.
- O'Brien, John T. (2014). "Clinical Significance of White Matter Changes". The American Journal of Geriatric Psychiatry. Elsevier BV. 22 (2): 133–137. doi:10.1016/j.jagp.2013.07.006. ISSN 1064-7481. PMID 24041523.
- Hirono, Nobutsugu; Kitagaki, Hajime; Kazui, Hiroaki; Hashimoto, Mamoru; Mori, Etsuro (2000). "Impact of White Matter Changes on Clinical Manifestation of Alzheimer's Disease". Stroke. Ovid Technologies (Wolters Kluwer Health). 31 (9): 2182–2188. doi:10.1161/01.str.31.9.2182. ISSN 0039-2499.
- Reddick, Wilburn E.; Shan, Zuyao Y.; Glass, John O.; Helton, Susan; Xiong, Xiaoping; Wu, Shengjie; Bonner, Melanie J.; Howard, Scott C.; Christensen, Robbin; Khan, Raja B.; Pui, Ching-Hon; Mulhern, Raymond K. (2006-02-15). "Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia". Cancer. Wiley. 106 (4): 941–949. doi:10.1002/cncr.21679. ISSN 0008-543X. PMC 2396784. PMID 16411228.
- Tasman, Allan (2015). Psychiatry (in Welsh). West Sussex, England: Wiley Blackwell. ISBN 978-1-118-84549-3. OCLC 903956524.
- Fields, R. Douglas (2008-06-05). "White matter in learning, cognition and psychiatric disorders". Trends in Neurosciences. Elsevier BV. 31 (7): 361–370. doi:10.1016/j.tins.2008.04.001. ISSN 0166-2236. PMC 2486416. PMID 18538868.
- Handbook of the Psychology of Aging. Elsevier. 2016. doi:10.1016/c2012-0-07221-3. ISBN 978-0-12-411469-2.
- Castronovo, Vincenza; Scifo, Paola; Castellano, Antonella; Aloia, Mark S.; Iadanza, Antonella; Marelli, Sara; Cappa, Stefano F.; Strambi, Luigi Ferini; Falini, Andrea (2014-09-01). "White Matter Integrity in Obstructive Sleep Apnea before and after Treatment". Sleep. Oxford University Press (OUP). 37 (9): 1465–1475. doi:10.5665/sleep.3994. ISSN 0161-8105. PMC 4153061. PMID 25142557.
- Canessa, Nicola; Castronovo, Vincenza; Cappa, Stefano F.; Aloia, Mark S.; Marelli, Sara; Falini, Andrea; Alemanno, Federica; Ferini-Strambi, Luigi (2011-05-15). "Obstructive Sleep Apnea: Brain Structural Changes and Neurocognitive Function before and after Treatment". American Journal of Respiratory and Critical Care Medicine. American Thoracic Society. 183 (10): 1419–1426. doi:10.1164/rccm.201005-0693oc. ISSN 1073-449X. PMID 21037021.
- Chen, Hsiu-Ling; Lu, Cheng-Hsien; Lin, Hsin-Ching; Chen, Pei-Chin; Chou, Kun-Hsien; Lin, Wei-Ming; Tsai, Nai-Wen; Su, Yu-Jih; Friedman, Michael; Lin, Ching-Po; Lin, Wei-Che (2015-03-01). "White Matter Damage and Systemic Inflammation in Obstructive Sleep Apnea". Sleep. Oxford University Press (OUP). 38 (3): 361–370. doi:10.5665/sleep.4490. ISSN 0161-8105. PMC 4335530. PMID 25325459.
- Ferini-Strambi, L.; Marelli, S.; Galbiati, A.; Castronovo, C. (2013). "Effects of continuous positive airway pressure on cognitition and neuroimaging data in sleep apnea". International Journal of Psychophysiology. Elsevier BV. 89 (2): 203–212. doi:10.1016/j.ijpsycho.2013.03.022. ISSN 0167-8760. PMID 23570950.
Structural changes have been demonstrated in brain regions including areas that regulate memory and executive function (e.g., frontal cortex, anterior cingulate, and hippocampus). however, not to apply too much valence to imaging findings in this population, as imaging studies are limited in their ability to assess the effects of OSA for several reasons. For example, hypertension can affect imaging outcomes and it is well known that hypertension is highly prevalent among OSA patients. Despite these limitations, the current cohort of studies suggests that imaging can be utilized to detect important, and even clinically relevant, changes associated with treatment among individuals affected by OSA.
- ALOIA, MARK S.; SWEET, LAWRENCE H.; JERSKEY, BETH A.; ZIMMERMAN, MOLLY; TODD ARNEDT, JOHN; MILLMAN, RICHARD P. (2009). "Treatment effects on brain activity during a working memory task in obstructive sleep apnea" (PDF). Journal of Sleep Research. Wiley. 18 (4): 404–410. doi:10.1111/j.1365-2869.2009.00755.x. ISSN 0962-1105. PMID 19765205.
- Assaf, Yaniv; Pasternak, Ofer (2007). "Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review". Journal of Molecular Neuroscience. 34 (1): 51–61. doi:10.1007/s12031-007-0029-0. PMID 18157658.
- Scholz, Jan; Klein, Miriam C; Behrens, Timothy E J; Johansen-Berg, Heidi (2009). "Training induces changes in white-matter architecture". Nature Neuroscience. 12 (11): 1370–1. doi:10.1038/nn.2412. PMC 2770457. PMID 19820707.
- "White Matter Matters". Dolan DNA Learning Center. Archived from the original on 2009-11-12. Retrieved 2009-10-19.
- Sampaio-Baptista, C.; Khrapitchev, A. A.; Foxley, S.; Schlagheck, T.; Scholz, J.; Jbabdi, S.; Deluca, G. C.; Miller, K. L.; Taylor, A.; Thomas, N.; Kleim, J.; Sibson, N. R.; Bannerman, D.; Johansen-Berg, H. (2013). "Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination". Journal of Neuroscience. 33 (50): 19499–503. doi:10.1523/JNEUROSCI.3048-13.2013. PMC 3858622. PMID 24336716.
External links
| Wikimedia Commons has media related to White matter. |
Further reading
- White Matter Atlas
- WebMD (2009). "white matter". Webster's New World Medical Dictionary (3rd ed.). Houghton Mifflin Harcourt. p. 456. ISBN 978-0-544-18897-6.
- "White Matter Changes with Normal Aging". Surgical Planning Laboratory. Retrieved 2019-03-19.
- Eileen L'ders, Helmuth Steinmetz, Lutz Jncke (8 July 2002). "Brain size and grey matter volume in the healthy human brain" (PDF). Cognitive Neuroscience and Neuropsychology. Institute of Experimental and General Psychology, Otto-von-Guericke-University-Magdeburg Department of Neurology, Johann-Wolfgang Goethe University Frankfurt am Main, Germany; Institute of Psychology, Neuropsychology, University Zˇrich, Treichlerstr.10,CH- 8032 Zˇrich, Switzerland. 13 (17): 2371–4. doi:10.1097/01.wnr.0000049603.85580.da (inactive 2019-08-20). PMID 12488829.CS1 maint: uses authors parameter (link)
- Fields, R. D. (2010-11-04). "Change in the Brain's White Matter". Science. American Association for the Advancement of Science (AAAS). 330 (6005): 768–769. doi:10.1126/science.1199139. ISSN 0036-8075. PMC 3201847. PMID 21051624.
- Filley, Christopher M.; Fields, R. Douglas (2016-08-10). "White matter and cognition: making the connection". Journal of Neurophysiology. American Physiological Society. 116 (5): 2093–2104. doi:10.1152/jn.00221.2016. ISSN 0022-3077. PMC 5102321. PMID 27512019.
- Budday, S; Nay, R; de Rooij, R; Steinmann, P; Wyrobek, T; Ovaert, TC; Kuhl, E (2015-03-02). "Mechanical properties of gray and white matter brain tissue by indentation". Journal of the Mechanical Behavior of Biomedical Materials. 46: 318–330. doi:10.1016/j.jmbbm.2015.02.024. PMC 4395547. PMID 25819199.