Obstructive sleep apnea
Obstructive sleep apnea (OSA) is the most common type of sleep apnea and is characterised by repeated episodes of complete or partial obstructions of the upper airway during sleep, despite the effort to breathe, and is usually associated with a reduction in blood oxygen saturation. In the Obstructive Sleep Apnea-Hypopnea Syndrome, the episodes of decreased breathing are called “hypopnea” and its definition requires a ≥30% drop in flow for 10 seconds or longer, associated with ≥3% oxygen desaturation. The episodes of breathing cessations are called “apneas” (literally, “without breath”) and to be defined, a ≥90% drop in flow for 10 seconds or longer must be assessed and associated with ≥3% oxygen desaturation, or an arousal.[1]
| Obstructive sleep apnea | |
|---|---|
| Other names | Obstructive sleep apnoea |
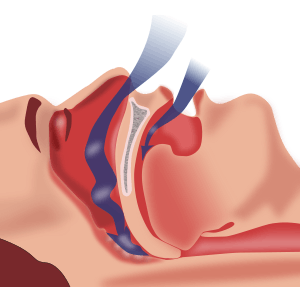 | |
| Obstructive sleep apnea: As soft tissue falls to the back of the throat, it impedes the passage of air (blue arrows) through the trachea. | |
| Specialty | Sleep medicine |
In the third edition of the International Classification of Sleep Disorders (ICSD-3), Obstructive Sleep Apnea is classified amongst the Sleep Related Breathing Disorders and is divided in two categories, namely adult OSA and pediatric OSA.[2] Obstructive Sleep Apnea is differentiated from Central Sleep Apnea (CSA) which is characterized by changes in the respiratory cycle during sleep but without the effort to breath during the apnea.[3] The respiratory effort must then be assessed in order to correctly classify the apnea as obstructive given the specificity of the diaphragmatic activity in this condition:[4] the inspiratory effort is continued or increased through the entire episode of absent airflow.[5] When hypopneas are present alongside apneas, the term Obstructive Sleep Apnea-Hypopnea is used and when it is associated with daytime sleepiness and other daytime symptoms, it is called Obstructive Sleep Apnea-Hypopnea Syndrome.[6] To be categorized as Obstructive, the hypopnea must meet one or more of the following symptoms: (1) snoring during the event, (2) increased oronasal flow flattening, and/or (3) thoraco-abdominal paradoxical respiration during the event [5] If none of them are present during the event, it is categorized as central hypopnea.
Individuals with OSA are rarely aware of difficulty breathing, even upon awakening. It is often recognized as a problem by others who observe the individual during episodes or is suspected because of its effects on the body. OSA is commonly accompanied with snoring. The terms obstructive sleep apnea syndrome or obstructive sleep apnea–hypopnea syndrome are used to refer to OSA when it is associated with symptoms during the daytime (e.g. excessive daytime sleepiness, decreased cognitive functions).[7][8] Symptoms may be present for years or even decades without identification, during which time the individual may become conditioned to the daytime sleepiness and fatigue associated with significant levels of sleep disturbance. Individuals who generally sleep alone are often unaware of the condition, without a regular bed-partner to notice and make them aware of the signs.
As the muscle tone of the body ordinarily relaxes during sleep, and the airway at the throat is composed of walls of soft tissue, which can collapse, it is not surprising that breathing can be obstructed during sleep. Although a minor degree of OSA is considered to be within the bounds of normal sleep, and many individuals experience episodes of OSA at some point in life, a small percentage of people have chronic, severe OSA.
Many people experience episodes of OSA for only a short period. This can be the result of an upper respiratory infection that causes nasal congestion, along with swelling of the throat, or tonsillitis that temporarily produces very enlarged tonsils. The Epstein-Barr virus, for example, is known to be able to dramatically increase the size of lymphoid tissue during acute infection, and OSA is fairly common in acute cases of severe infectious mononucleosis. Temporary spells of OSA syndrome may also occur in individuals who are under the influence of a drug (such as alcohol) that may relax their body tone excessively and interfere with normal arousal from sleep mechanisms.
Signs and symptoms
Common symptoms of OSA include unexplained daytime sleepiness, restless sleep, and loud snoring (with periods of silence followed by gasps). Less common symptoms are morning headaches; insomnia; trouble concentrating; mood changes such as irritability, anxiety and depression; forgetfulness; increased heart rate and/or blood pressure; decreased sex drive; unexplained weight gain; increased urination and/or nocturia; frequent heartburn or gastroesophageal reflux disease; and heavy night sweats. Whereas the vast majority of patients with obstructive sleep apnea exhibit snoring, a minority (20-25%) of patients with central sleep apnea do not snore.
Adults
The hallmark symptom of OSA syndrome in adults is excessive daytime sleepiness. Typically, an adult or adolescent with severe long-standing OSA will fall asleep for very brief periods in the course of usual daytime activities if given any opportunity to sit or rest. This behavior may be quite dramatic, sometimes occurring during conversations with others at social gatherings.
The hypoxia (absence of oxygen supply) related to OSA may cause changes in the neurons of the hippocampus and the right frontal cortex. Research using neuro-imaging revealed evidence of hippocampal atrophy in people suffering from OSA. They found that OSA can cause problems in mentally manipulating non-verbal information, in executive functions and working memory. This repeated brain hypoxia is also considered to be a cause of Alzheimer's disease.[9]
Diagnosis of obstructive sleep apnea is significantly more common among people in relationships, who are alerted to their condition by being informed by their sleeping partner since individuals with obstructive sleep apnea are often unaware of the condition. There is a stigma associated with loud snoring, and it is not considered a feminine trait. Consequently, females are less likely to be told by their partners that they snore, or to admit it to themselves or doctors. Furthermore, CPAP (Continuous Positive Airway Pressure) machines are also perceived negatively by females, and less likely to be utilized to their full extent in this group.[10]
Children
Although this so-called "hypersomnolence" (excessive sleepiness) may also occur in children, it is not at all typical of young children with sleep apnea. Toddlers and young children with severe OSA instead ordinarily behave as if "over-tired" or "hyperactive." Adults and children with very severe OSA also differ in typical body habitus. Adults are generally heavy, with particularly short and heavy necks. Young children, on the other hand, are generally not only thin but may have "failure to thrive", where growth is reduced. Poor growth occurs for two reasons: the work of breathing is intense enough that calories are burned at high rates even at rest, and the nose and throat are so obstructed that eating is both tasteless and physically uncomfortable. OSA in children, unlike adults, is often caused by obstructive tonsils and adenoids and may sometimes be cured with tonsillectomy and adenoidectomy.
This problem can also be caused by excessive weight in children. In this case, the symptoms are more like the symptoms adults feel such as restlessness, exhaustion, etc. If adenotonsillar hypertrophy remains the most common cause of OSA in children,[11][12] obesity can also play a role in the pathophysiology of upper airway obstruction during sleep which can lead to OSA, making obese children more likely to develop the condition.[13] The recent epidemic increase of obesity prevalence has thus contributed to changes in the prevalence and in the characteristics of pediatric OSA,[14] the severity of OSA being proportional to the degree of obesity.[15][16] Obesity leads to the narrowing of upper airway structure due to fatty infiltration and fat deposits in the anterior neck region and cervical structures.[11][14] Alongside with the additional weight loading on the respiratory system, it increases the risk of pharyngeal collapsibility while reducing the intrathoracic volume and diaphragm excursion.[14] Moreover, excessive daytime sleepiness resulting from sleep fragmentation can decrease physical activity and thus lead to weight gain (by sedentary habits or increased food intake to overcome somnolence).[17] The obesity-related obstruction of upper airway structure has led some authors to distinguish between two types of OSA in children:[14][13] type I is associated with marked lymphadenoid hypertrophy without obesity and type II is first associated with obesity and with milder upper airway lymphadenoid hyperplasia. The two types of OSA in children can results in different morbidities and consequences.[13] Studies have shown that weight loss in obese adolescents can reduce sleep apnea and thus the symptoms of OSA.[11][16]
Children with OSA may experience learning and memory deficits and OSA has also been linked to lowered childhood IQ scores.[18]
Causes
Most cases of OSA are believed to be caused by:
- Old age (natural or premature)
- Brain injury (temporary or permanent)
- Decreased muscle tone. This can be caused by drugs or alcohol, or it can be caused by neurological problems or other disorders. Some people have more than one of these issues. There is also a theory that long-term snoring might induce local nerve lesions in the pharynx in the same way as long-term exposure to vibration might cause nerve lesions in other parts of the body. Snoring is a vibration of the soft tissues of the upper airways, and studies have shown electrophysiological findings in the nerves and muscles of the pharynx indicating local nerve lesions.
- Increased soft tissue around the airway (sometimes due to obesity)
- Structural features that give rise to a narrowed airway.
Some adults with OSA are obese. Obese adults show an increase in pharyngeal tissue which cause respiratory obstruction during sleep.[19] Adults with normal body mass indices (BMIs) often have decreased muscle tone causing airway collapse and sleep apnea. Sleeping on the supine position is also a risk factor for OSA. The supine sleeping position generates mandibular retraction and tongue collapse which constitutes an anatomical basis for respiratory obstruction during sleep.
OSA and recurrent tonsillitis (RT) are different in their mechanism and outcome.[20]
Risk factors
Old age is often accompanied by muscular and neurological loss of muscle tone of the upper airway. Decreased muscle tone is also temporarily caused by chemical depressants; alcoholic drinks and sedative medications being the most common. The permanent premature muscular tonal loss in the upper airway may be precipitated by traumatic brain injury, neuromuscular disorders, or poor adherence to chemical and or speech therapy treatments.
Individuals with decreased muscle tone and increased soft tissue around the airway, and structural features that give rise to a narrowed airway are at high risk for OSA. Men, in which the anatomy is typified by increased mass in the torso and neck, are at increased risk of developing sleep apnea, especially through middle age and later. Women suffer typically less frequently and to a lesser degree than do men, owing partially to physiology, but possibly also to differential levels of progesterone. Prevalence in post-menopausal women approaches that of men in the same age range. Women are at greater risk for developing OSA during pregnancy.[21]
OSA also appears to have a genetic component; those with a family history of it are more likely to develop it themselves. Lifestyle factors such as smoking may also increase the chances of developing OSA as the chemical irritants in smoke tend to inflame the soft tissue of the upper airway and promote fluid retention, both of which can result in narrowing of the upper airway. Cigarette may also have an impact due to a decline of blood nicotine levels, which alters sleep stability.[8] Smokers thus show a higher risk to develop OSA, but the effect of cigarette on increased OSA is reversible with the cessation of smoking.[8] Children exposed to cigarette smoke may also develop OSA as the lymphadenoid tissue will proliferate excessively in contact of the irritants.[22] An individual may also experience or exacerbate OSA with the consumption of alcohol, sedatives, or any other medication that increases sleepiness as most of these drugs are also muscle relaxants.[23] Allergic rhinitis and asthma have also been shown to be implicated in the increased prevalence of adenotonsillar hypertrophy and OSA.[24][25]
Craniofacial syndromes
There are patterns of unusual facial features that occur in recognizable syndromes. Some of these craniofacial syndromes are genetic, others are from unknown causes. In many craniofacial syndromes, the features that are unusual involve the nose, mouth, and jaw, or resting muscle tone, and put the individual at risk for OSA syndrome.
Down syndrome is one such syndrome. In this chromosomal abnormality, several features combine to make the presence of obstructive sleep apnea more likely. The specific features of Down syndrome that predispose to obstructive sleep apnea include relatively low muscle tone, narrow nasopharynx, and large tongue. Obesity and enlarged tonsils and adenoids, conditions that occur commonly in the western population, are much more likely to be obstructive in a person with these features than without them. Obstructive sleep apnea does occur even more frequently in people with Down syndrome than in the general population. A little over 50% of all people with Down syndrome suffer from obstructive sleep apnea,[26] and some physicians advocate routine testing of this group.[27]
In other craniofacial syndromes, the abnormal feature may actually improve the airway, but its correction may put the person at risk for obstructive sleep apnea after surgery when it is modified. Cleft palate syndromes are such an example. During the newborn period, all humans are obligate nasal breathers. The palate is both the roof of the mouth and the floor of the nose. Having an open palate may make feeding difficult, but generally, does not interfere with breathing, in fact, if the nose is very obstructed, then an open palate may relieve breathing. There are a number of clefting syndromes in which the open palate is not the only abnormal feature; additionally, there is a narrow nasal passage - which may not be obvious. In such individuals, closure of the cleft palate – whether by surgery or by a temporary oral appliance, can cause the onset of obstruction.
Skeletal advancement in an effort to physically increase the pharyngeal airspace is often an option for craniofacial patients with upper airway obstruction and small lower jaws (mandibles). These syndromes include Treacher Collins syndrome and Pierre Robin sequence. Mandibular advancement surgery is often just one of the modifications needed to improve the airway, others may include reduction of the tongue, tonsillectomy or modified uvulopalatoplasty.
Post-operative complication
OSA can also occur as a serious post-operative complication that seems to be most frequently associated with pharyngeal flap surgery as compared to other procedures for the treatment of velopharyngeal inadequacy (VPI).[28] In OSA, recurrent interruptions of respiration during sleep are associated with temporary airway obstruction. Following pharyngeal flap surgery, depending on size and position, the flap itself may have an "obturator" or obstructive effect within the pharynx during sleep, blocking ports of airflow and hindering effective respiration.[29][30] There have been documented instances of severe airway obstruction, and reports of post-operative OSA continues to increase as healthcare professionals (i.e. physicians, speech language pathologists) become more educated about this possible dangerous condition.[31] Subsequently, in clinical practice, concerns of OSA have matched or exceeded interest in speech outcomes following pharyngeal flap surgery.
The surgical treatment for velopalatal insufficiency may cause obstructive sleep apnea syndrome. When velopalatal insufficiency is present, air leaks into the nasopharynx even when the soft palate should close off the nose. A simple test for this condition can be made by placing a tiny mirror on the nose, and asking the subject to say "P". This p sound, a plosive, is normally produced with the nasal airway closes off - all air comes out of the pursed lips, none from the nose. If it is impossible to say the sound without fogging a nasal mirror, there is an air leak - reasonable evidence of poor palatal closure. Speech is often unclear due to inability to pronounce certain sounds. One of the surgical treatments for velopalatal insufficiency involves tailoring the tissue from the back of the throat and using it to purposefully cause partial obstruction of the opening of the nasopharynx. This may actually cause OSA syndrome in susceptible individuals, particularly in the days following surgery, when swelling occurs (see below: Special Situation: Anesthesia and Surgery).
Finally, patients with OSA are at an increased risk of many perioperative complications when they are present for surgery, even if the planned procedure is not on the head and neck. Guidelines intended to reduce the risk of perioperative complications have been published.[32]
Consequences
In adults
There are 3 levels of consequences: physiologic, intermediate and clinical.[33] The physiologic consequences contain hypoxia, sleep fragmentation, autonomic nervous system dysregulation or hyperoxia.[33] The intermediate results regroup inflammation, pulmonary vasoconstriction, general metabolic dysfunction, oxidation of proteins and lipids or increased adiposity.[33] The clinical repercussions are composed by pulmonary hypertension, accidents, obesity, diabetes, different heart diseases or hypertension.[33]
In children
Obstructive Sleep Apnea is the most common Sleep-Disordered Breathing (SDB) and affects up to 11% of children born at term - it is even more common (3 to 6 times more) in children born pre-term.[34] As a SDB, OSA in children can lead to several adverse consequences, also in the long-term with consequences lasting into adulthood.[11] The implications of OSA in children are complex and cover a large scope of consequences: when it is left untreated, OSA can lead to morbidity affecting many different domains of life (organs, body systems, behavioural disturbance, depression, decreased quality of life, etc.).[22] Therefore, nocturnal symptoms indicating the presence of OSA (e.g. snoring, gasping, restless sleep and excessive energy used to breath during sleep) are associated with daytime symptoms such as concentration and learning difficulties and irritability, neurocognitive development impairment, decreased school performance and behaviour difficulties.[11] For example, SDB such as OSA contribute to hyperactive behaviour that can lead to the diagnosis and treatment of Attention deficit hyperactivity disorder (ADHD). However, once the SDB is treated, the hyperactive behaviour can improve, and the treatment can be stopped.[35] Obesity also has an impact on the consequences of OSA and lead to different manifestations or severity.[22] Studies have shown that, contrary to adults, children with obstructive sleep-disordered breathing are able to maintain cerebral oxygenation. However, the condition still has effects on the brain and can lead to adverse neurocognitive and behavioural sequelae. It is particularly concerning as those consequences happen while the brain is still developing.[34] The degree to which the sleep is disturbed and fragmented has been significantly linked to the severity of the consequences, the latter having the possibility to decrease once the sleep is improved.[36] It is more the disruption of sleep processes than the total amount of sleep the child experience that generates the adverse consequences on the child’s daytime functioning;[36] it contributes to the hyperactivity for example.[35]
Neurocognitive and behavioural Consequences
Nocturnal sleep fragmentation has been linked to neurocognitive impairments, therefore, the identification of SDB such as OSA is crucial in children, those impairments having the possibility to be reversible with the appropriate treatment for the sleep disorder.[37] The neurocognitive and behavioural dysfunctions commonly present in children with OSA include the following: hyperactivity, impulsivity, aggressive behaviours,[22][36] low social and communicational abilities and reduced adaptive skills.[11] Children with OSA commonly show cognitive deficits, resulting in attention and concentration difficulties, as well as lower academic performance and IQ.[11][36] Poor academic performances have been linked to OSA and suggested to result from cortical and sympathetic arousals and hypoxemia which affects memory consolidation.[38] A study with Indian children affected with OSA has shown poor school grades, including mathematics, science, language and physical education. This study allowed to see the overall impact of OSA on learning abilities associated with language or numeracy skills, and physical development.[38] It has been suggested that the deficits in academic performance related to OSA could be mediated through reduced executive functions and/or language skills,[39] those domains contributing highly to learning abilities and behaviour. The deficits in school performance can nevertheless be improved if adenotonsillectomy is performed on children to treat the OSA.[39] It is thus crucial to identify the OSA for children with school difficulties; many cases remaining unnoticed.[39] As studies have shown that learning skills and behaviours can be improved if the OSA is treated,[11][36] the neurocognitive and behavioural deficits are thus at least partly reversible.[22] This reversible dimension has been postulated to be negatively correlated to the duration of the symptoms, which would mean that the longer the OSA is left untreated, the less reversible are the consequences.[36]
Somatic and metabolic consequences
Similarly to adults, OSA in children is linked to a higher risk for cardiovascular morbidities,[22][36] due to increased sympathetic activity and impaired cardiac autonomic control.[34] Amongst the cardiovascular dysfunctions resulting from OSA, we can find systemic hypertension[36] and blood pressure dysregulation[11][40] (elevated blood pressure, or variability of the blood pressure for example[34]). The variability of the blood pressure has been shown to be correlated with the severity of the symptoms such as the frequency of the apnea and hypopnea.[40] Pulmonary hypertension is also common amongst the cardiovascular problems resulting from OSA.[11] Children with obstructive sleep-disordered breathing also show a faster heart rate during wakefulness and during sleep.[40] In adult patients, OSA has been shown to be associated with insulin resistance.[41] In children, metabolic consequences of OSA are complicated to assess as they can also be associated to puberty and/or obesity (if present).[11] However, when OSA is associated with obesity, the interaction of the two conditions can lead to metabolic disturbances such as insulin resistance and altered lipidemia,[22] liver disease, abdominal adiposity and metabolic syndrome. Obesity interact with those effects.[40]
Nocturnal enuresis
Children with OSA also show a higher risk for nocturnal enuresis[11][42] and it is hypothesized to be caused by an excessive production of urine,[38][43] impaired performance of the bladder and urethra[44] or an inability to suppress the nocturnal bladder contraction, due to a failure to arouse.[43][44] The risk for nocturnal enuresis increases with the severity of the sleep-disordered breathing: the more respiratory events per hour of sleep, the higher is the risk for nocturnal enuresis.[44] Obesity may also play a role as it is associated with OSA and with nocturnal diuresis (due to unhealthy diet). The interaction between OSA and obesity might thus result in nocturnal enuresis.[43] Considering the high prevalence of nocturnal enuresis amongst children with sleep-disordered breathing, it is important to consider the latter in the differential diagnosis of nocturnal enuresis as the treatment of the sleep disorder might have a favourable therapeutic effect on the enuresis.[45][42][44] For example, an adenotonsillectomy performed to reduce OSA has a positive impact on nocturnal enuresis.[45][38] A study has shown that this surgery has 60-75% chance to resolve the nocturnal enuresis completely, and 80-85% chance to reduce its symptoms alongside others symptoms of OSA.[42]
Other consequences
Contrary to adults, Excessive Daytime Sleepiness (EDS) is not the most commonly reported symptoms in children with OSA.[37] However, using objective questionnaires, it is possible to notice that the frequency of EDS in children is higher than what is reported by the parents or caretakers (40-50%).[36] And the risk for EDS is even increased when OSA is associated with obesity.[36][22] Due to all the consequences and symptoms it generates, OSA in children lead to a significant decrease in the quality of life,[36] the decrease being even higher when obesity is present.[22] The quality of life can however be improved with the treatment of OSA.[22] SBD have also been linked to a higher rate of internalizing disorders such as anxiety and depression.[46] Indeed, depressive symptoms have shown to be higher in children with OSA,[46] especially in males.[47] Once again, the severity of depressive symptoms is positively correlated with the severity of the SBD.[46] It also interacts with obesity as obese children have higher risk to show depressive symptoms and obesity can cause OSA.[47] The link can also go the other way around with the depression inducing obesity (due to overeating) which worsen the OSA. Adenotonsillectomy can decrease the intensity of the depressive symptoms.[47] Other consequences of a disturbed sleep in children with OSA comprise anhedonia[48][46] increased fatigue and decreased interest in daily activities, which in turn can affect the child’s social relationships.[22]
In adults
While there are some similarities between adults and children, OSA does not have the same consequences in both populations.[49] Examples of similarities are the snoring – which is the most common complaint in both pediatric OSA and OSA in adults[49] –, variability of blood pressure and cardiovascular morbidities.[8] A major difference is the excessive daytime sleepiness (EDS) which is commonly reported in adult OSA,[50] while it is not very common in pediatric OSA.[49] Nevertheless, OSA in adults also implies a large scope of adverse and serious consequences,[51] the latter leading to higher mortality amongst OSA patients.[52] Those consequences are even worsen by common morbidities such as obesity.[53]
Neurocognitive Consequences
Similarly to children, OSA affects cognitive functions in adults.[49] Meta-analysis have shown that the most common cognitive impairments happen in the domains of attention, verbal and visual delayed long-term memory,[54] visuospatial/constructional abilities and executive functions,[55] such as mental flexibility.[54] The executive functioning – mainly dominated by the prefrontal cortex[56] – being significantly impaired in patients with OSA, it is believed that the prefrontal region and its connectivity are affected by sleep disorders.[57] Regarding memory deficits, verbal memory is significantly impaired as patients show difficulties in recalling verbal information immediately as well as with a delay. While meta-analysis have shown no deficits in retention of information for patients with OSA, those impairment in verbal memory may be linked to problems in encoding information.[58] This deficit in encoding of information is also noticed in visuo-spatial memory; however, the visual memory seems to be intact in OSA patients.[58] The cognitive impairments have been suggested to be resulting from sleep fragmentation and sleep deprivation, as well as the excessive daytime sleepiness associated with them.[55] More precisely, attention and memory deficits seem to result from sleep fragmentation, while deficits in global cognitive function (executive function, psychomotor function, language abilities) are more related to hypoxia and/or hypercarbia which accompanies the obstructive events during sleep.[55][59] However, no consistent correlation has been found between the degree of cognitive impairment and the severity of the sleep disturbance or hypoxia.[59][58] These impairments may improve with an effective treatment for OSA, such as continuous positive airway pressure (CPAP) therapy.[55]
Behavioural consequences
The hyperactivity and difficulties in emotional regulation found in children patients are not reported in adults.[49] OSA in adults is nevertheless associated with personality changes and automatic behaviour.[50] The biggest impact of OSA is the excessive daytime sleepiness (EDS), reported in approximately 30% of OSA patients.[60] EDS can be caused by the disturbance of sleep quality, the insufficient sleep duration or the sleep fragmentation[61] and it is responsible for further complications as it may lead to depressive symptoms,[62] impairments of social life and decreased effectiveness at work.[63][64] Studies have shown that those consequences of EDS can be improved following a CPAP treatment.[65]
Physiological and metabolic consequences
OSA in adults is associated with a higher risk for cardiovascular morbidities, diabetes, hypertension, coronary artery disease and stroke[49][50] – OSA might have a role in the etiology of these conditions. Those conditions may lead to increased mortality[49][50] that an appropriate treatment for OSA may reduce.[50] OSA is often linked with hypertension as it induces an increase in sympathetic activity that can lead to the elevation of blood pressure. The OSA-related hypercapnia has been suggested to be related to this development of hypertension.[66] Treating the OSA may prevent the development of hypertension.[67] The relationship between OSA and excess body weight is complex as obesity is more prevalent amongst OSA patients but can also be a risk factor for the development of OSA[50] – it accounts for 58% of adult cases.[68] Thus, both OSA and obesity (when present) may work synergistically and lead to hyperlipidaemia, diabetes, insulin resistance and other symptoms of the metabolic syndrome.[50][8] The metabolic syndrome itself is often associated with OSA: 74-85% of OSA patients are diagnosed with it. CPAP therapy can lead to an improvement of some of the cardiovascular component of the metabolic syndrome[67] while weight loss is also recommended for its positive effects on OSA consequences and metabolic dysfunctions.[68][69] An intervention comprising exercise and diet is thus effective for the treatment of OSA as it positively impacts the severity of both obesity symptoms and OSA symptoms.[70]
Psychological consequences
Sleep is of major importance for psychological and emotional health.[71] As it is greatly impaired in OSA, this condition is associated with mood disorders[72] and several psychological outcomes,[71] especially depression and anxiety.[73] Therefore, psychological disorders are commonly observed in OSA patients who show a higher prevalence of psychological distress, mostly due to the impaired sleep quality and structure and the repeated episodes of hypoxia.[71][74] The presence of psychological disorders may also worsen the sleep disorders which implies that the psychopathology may either be a factor or a consequence of the OSA.[73] The adverse consequences of OSA such as cardiovascular comorbidities and metabolic disturbances also play a role on the development of mental disorders,[73] and patients suffering from chronic condition have also been reported to have higher risk to experience depression.[72] In OSA patients suffering from psychiatric disorders, it is crucial to treat the OSA as it may reduce the psychiatric symptoms.[75] Some cases of OSA are caused by nasal obstruction which has also been related to psychological problems due to an altered ratio of calcium and magnesium in brain cells. Nasal obstruction can thus aggravate the psychological health of OSA patients. Nasal surgery for those patients might decrease the OSA severity and improve the psychological symptoms.[74]
Other Consequences
Untreated OSA also leads to a decreased quality of life, difficulties in social functioning,[50] occupational problems and accidents[57] and a greatly increased rate of vehicle accidents.[76][50][77] Those serious outcomes of OSA are mostly related to the excessive daytime sleepiness resulting from the sleep fragmentation and highlight the need to provide the patients with appropriate treatment.[50] Effective treatment majorly improves those adverse consequences, including quality of life.[50] OSA patients also frequently reports pain disorders such as headache or fibromyalgia, OSA patients showing an increased pain intensity alongside a decreased pain tolerance.[77]
Pathophysiology
The normal sleep/wake cycle in adults is divided into REM (rapid eye movement) sleep, non-REM (NREM) sleep, and wakefulness. NREM sleep is further divided into Stages 1, 2 and 3 NREM sleep. The deepest stage (stage 3 of NREM) is required for the physically restorative effects of sleep, and in pre-adolescents, this is the period of release of human growth hormone. NREM stage 2 and REM, which combined are 70% of an average person's total sleep time, are more associated with mental recovery and maintenance. During REM sleep, in particular, muscle tone of the throat and neck, as well as the vast majority of all skeletal muscles, is almost completely attenuated, allowing the tongue and soft palate/oropharynx to relax, and in the case of sleep apnea, to impede the flow of air to a degree ranging from light snoring to complete collapse. In the cases where airflow is reduced to a degree where blood oxygen levels fall, or the physical exertion to breathe is too great, neurological mechanisms trigger a sudden interruption of sleep, called a neurological arousal. These arousals rarely result in complete awakening but can have a significant negative effect on the restorative quality of sleep. In significant cases of OSA, one consequence is sleep deprivation due to the repetitive disruption and recovery of sleep activity. This sleep interruption in stage 3 (also called slow-wave sleep), and in REM sleep, can interfere with normal growth patterns, healing, and immune response, especially in children and young adults.
Diagnosis
Diagnosis of OSA is often based on a combination of patient history and tests (lab- or home-based). These tests range, in decreasing order of cost, complexity and tethering of the patient (number and type of channels of data recorded), from lab-attended full polysomnography ("sleep study") down to single-channel home recording. In the USA, these categories are associated with insurance classification from Type I down to Type IV.[78] Reimbursement rules vary among European countries.[79] In a systematic review of published evidence, the United State Preventive Services Task Force in 2017 concluded that there was uncertainty about the accuracy or clinical utility of all potential screening tools for OSA,[80] and recommended that current evidence is insufficient to assess the balance of benefits and harms of screening for OSA in asymptomatic adults.[81]
The diagnosis of OSA syndrome is made when the patient shows recurrent episodes of partial and complete collapse of the upper airway during sleep resulting in hypopnea and apneas [82] – hypopnea being the reduction of airflow and apnea its complete cessation, both despite active efforts to breathe.[83] To define the severity of the condition, the Apnea-Hypopnea Index (AHI) or the Respirratory Distrubance Index (RDI) are used. While the AHI measures the mean number of apneas and hypopneas per hour of sleep, the RDI adds to this measure the respiratory effort-related arousals (RERAs).[84] The OSA syndrome is thus diagnosed if AHI > 5 episodes per hour and results in daytime sleepiness and fatigue or when RDI ≥ 15 independently of the symptoms.[85] According to the American Association of Sleep Medicine, daytime sleepiness is determined as mild, moderate and severe depending on its impact on social life. Daytime sleepiness can be assessed with the Epworth Sleepiness Scale (ESS), a self-reported questionnaire on the propensity to fall asleep or doze off during daytime.[86] Screening tools for OSA itself comprise the STOP questionnaire, the Berlin questionnaire and the STOP-BANG questionnaire which has been reported as being a very powerful tool to detect OSA.[87][88]
Criteria
According to the International Classification of Sleep Disorders, there are 4 types of criteria. The first one concerns sleep - excessive sleepiness, nonrestorative sleep, fatigue or insomnia symptoms. The second and third criteria are about respiration - waking with breath holding, gasping, or choking ; snoring, breathing interruptions or both during sleep. The last criterion revolved around medical issues as hypertension, coronary artery disease, stroke, heart failure, atrial fibrillation, type 2 diabetes mellitus, mood disorder or cognitive impairment. Two levels of severity are distinguished, the first one is determined by a polysomnography or home sleep apnea test demonstrating 5 or more predominantly obstructive respiratory events per hour of sleep and the higher levels is determined by 15 or more events. If the events are present less than 5 times per hour, no obstructive sleep apnea is diagnosed.[89]
Polysomnography
| AHI | Rating |
|---|---|
| <5 | Normal |
| 5-15 | Mild |
| 15-30 | Moderate |
| >30 | Severe |
Polysomnography in diagnosing OSA characterizes the pauses in breathing. As in central apnea, pauses are followed by a relative decrease in blood oxygen and an increase in the blood carbon dioxide. Whereas in central sleep apnea the body's motions of breathing stop, in OSA the chest not only continues to make the movements of inhalation, but the movements typically become even more pronounced. Monitors for airflow at the nose and mouth demonstrate that efforts to breathe are not only present but that they are often exaggerated. The chest muscles and diaphragm contract and the entire body may thrash and struggle.
An "event" can be either an apnea, characterised by complete cessation of airflow for at least 10 seconds, or a hypopnea in which airflow decreases by 50 percent for 10 seconds or decreases by 30 percent if there is an associated decrease in the oxygen saturation or an arousal from sleep.[90] To grade the severity of sleep apnea, the number of events per hour is reported as the apnea-hypopnea index (AHI). An AHI of less than 5 is considered normal. An AHI of 5-15 is mild; 15-30 is moderate and more than 30 events per hour characterizes severe sleep apnea.
Home oximetry
In patients who are at high likelihood of having OSA, a randomized controlled trial found that home oximetry (a non-invasive method of monitoring blood oxygenation) may be adequate and easier to obtain than formal polysomnography.[91] High probability patients were identified by an Epworth Sleepiness Scale (ESS) score of 10 or greater and a Sleep Apnea Clinical Score (SACS) of 15 or greater.[92] Home oximetry, however, does not measure apneic events or respiratory event-related arousals and thus does not produce an AHI value.
Treatment
Numerous treatment options are used in obstructive sleep apnea.[93] Avoiding alcohol and smoking is recommended,[94] as is avoiding medications that relax the central nervous system (for example, sedatives and muscle relaxants). Weight loss is recommended in those who are overweight. Continuous positive airway pressure (CPAP) and mandibular advancement devices are often used and found to be equally effective.[95][96] Physical training, even without weight loss, improves sleep apnea.[97] There is insufficient evidence to support widespread use of medications or surgery.[95]
Physical intervention
The most widely used current therapeutic intervention is positive airway pressure whereby a breathing machine pumps a controlled stream of air through a mask worn over the nose, mouth, or both. The additional pressure holds open the relaxed muscles. There are several variants:
- Continuous positive airway pressure (CPAP) is effective for both moderate and severe disease.[98] It is the most common treatment for obstructive sleep apnea.
- Variable positive airway pressure (VPAP) (also known as bilevel (BiPAP or BPAP)) uses an electronic circuit to monitor the patient's breathing, and provides two different pressures, a higher one during inhalation and a lower pressure during exhalation. This system is more expensive, and is sometimes used with patients who have other coexisting respiratory problems and/or who find breathing out against an increased pressure to be uncomfortable or disruptive to their sleep.
- Nasal EPAP, which is a bandage-like device placed over the nostrils that utilizes a person's own breathing to create positive airway pressure to prevent obstructed breathing.[99]
- Automatic positive airway pressure, also known as "Auto CPAP", incorporates pressure sensors and monitors the person's breathing.[100][101]
- A 5% reduction in weight among those with moderate to severe OSA may decrease symptoms similarly to CPAP.[102]
Oral appliances or splints are often preferred but may not be as effective as CPAP.[98] This device is a mouthguard similar to those used in sports to protect the teeth. It is designed to hold the lower jaw slightly down and forward relative to the natural, relaxed position. This position holds the tongue farther away from the back of the airway and may be enough to relieve apnea or improve breathing.
Many people benefit from sleeping at a 30-degree elevation of the upper body[103] or higher, as if in a recliner. Doing so helps prevent the gravitational collapse of the airway. Sleeping on a side as opposed to sleeping on the back is also recommended.[104][105][106]
Some studies have suggested that playing a wind instrument: may reduce snoring and apnea incidents.[107] This may be especially true of double reed instruments.[108]
Surgery
Surgical treatments to modify airway anatomy, known as sleep surgery, are varied and must be tailored to the specific airway obstruction needs of a patient. Surgery is not considered a frontline treatment for obstructive sleep apnea, as prospective, randomized, comparative clinical evidence against current front line treatments is lacking.[95][109] For those obstructive sleep apnea sufferers unable or unwilling to comply with front line treatment, a properly selected surgical intervention will be the result of considering an individual's specific anatomy and physiology, personal preference and disease severity.[93] There is little randomized clinical trial evidence for all types of sleep surgery.[95]
There are a number of different operations that may be performed including:
- Septoplasty is a corrective surgical procedure for Nasal septum deviation in which the nasal septum is straightened.
- Tonsillectomy and/or adenoidectomy in an attempt to increase the size of the airway.
- Removal or reduction of parts of the soft palate and some or all of the uvula, such as uvulopalatopharyngoplasty (UPPP) or laser-assisted uvulopalatoplasty (LAUP). Modern variants of this procedure sometimes use radiofrequency waves to heat and remove tissue.
- Turbinectomy is a surgical procedure in which all or some of the turbinate bones are removed to relieve nasal obstruction.
- Reduction of the tongue base, either with laser excision or radiofrequency ablation.
- Genioglossus advancement, in which a small portion of the lower jaw that attaches to the tongue is moved forward, to pull the tongue away from the back of the airway.
- Hyoid suspension, in which the hyoid bone in the neck, another attachment point for tongue muscles, is pulled forward in front of the larynx.
- Maxillomandibular advancement[110]
In the morbidly obese, a major loss of weight (such as what occurs after bariatric surgery) can sometimes cure the condition.
OSA in children is sometimes due to chronically enlarged tonsils and adenoids. Tonsillectomy and adenoidectomy are curative. The operation may be far from trivial, especially in the worst apnea cases, in which growth is retarded and abnormalities of the right heart may have developed. Even in these extreme cases, the surgery tends to cure not only the apnea and upper airway obstruction but allows normal subsequent growth and development. Once the high end-expiratory pressures are relieved, the cardiovascular complications reverse themselves. The postoperative period in these children requires special precautions (see "Surgery and obstructive sleep apnea syndrome" below).
Neurostimulation
For patients who cannot use a continuous positive airway pressure device, the U.S. Food and Drug Administration in 2014 granted pre-market approval for an upper airway stimulation system that senses respiration and delivers mild electrical stimulation to the hypoglossal nerve in order to increase muscle tone at the back of the tongue so it will not collapse over the airway. The device includes a handheld patient controller to allow it to be switched on before sleep and is powered by an implantable pulse generator, similar to one used for cardiac rhythm management. Approval for this active implantable neuromodulation device was preceded by a clinical trial whose results were published in the New England Journal of Medicine.[111][112]
Radiofrequency ablation
Radiofrequency ablation (RFA), which is conceptually analogous in some ways to surgery, uses low frequency (300 kHz to 1 MHz)[113] radio wave energy to target tissue, causing coagulative necrosis. RFA achieves its effects at 40 °C to 70 °C[114] unlike other electrosurgical devices which require 400 °C to 600 °C for efficacy.[115]
Subsequent evaluations of safety and efficacy have led to the recognition of RFA by the American Academy of Otolaryngology[109] as a somnoplasty treatment option in selected situations for mild to moderate OSA, but the evidence was judged insufficient for routine adoption by the American College of Physicians.[95]
RFA has some potential advantages in carefully selected medical settings, such as intolerance to the CPAP device. For example, when adherence is defined as greater than four hours of nightly use, 46% to 83% of patients with obstructive sleep apnea are non-adherent with CPAP[116] for a variety of reasons, including discomfort while sleeping.
RFA is usually performed in an outpatient setting, using either local anesthetics or conscious sedation anesthesia, the procedure itself typically lasting under 3 minutes. The targeted tissue, such as tongue or palate, is usually approached through the mouth without the need for incisions, although occasionally the target is approached through the neck using assisted imaging.[117] If the tongue is being targeted, this can be done from either dorsal or ventral side. Complications include ulceration, infection, nerve weakness or numbness and swelling. These complications occur in less than 1% of procedures.[113]
Medications
Evidence is insufficient to support the use of medications to treat obstructive sleep apnea.[95][118] This includes the use of fluoxetine, paroxetine, acetazolamide and tryptophan among others.[95][119]
Recent studies are trying to investigate cannabinoids as a treatment for OSA, especially dronabinol which is a synthetic form of THC (delta-9-tetrahydrocannabinol). Cannabis is known to influence sleep, for example it can reduce sleep onset latency, however, results are not consistent.[120] Studies about dronabinol have shown positive impact on the OSA, as they observed a reduced AHI (Apnea-Hypopnea Index) and an increased self-reported sleepiness (the objective sleepiness being unaffected).[121] However, more evidence are needed as many effects of those substances remain unknown, especially the effects of a long-term intake.[122] The effect on sleepiness and weight gain are particularly of concern.[123] Because of uncertainty about its effects and a lack of consistent evidence, the American Academy of Sleep Medicine does not approve the use of medical cannabis for the treatment of OSA.[124][122]
Prognosis
Stroke and other cardiovascular disease are related to OSA and those under the age of 70 have an increased risk of early death.[125] Persons with sleep apnea have a 30% higher risk of heart attack or death than those unaffected.[126] In severe and prolonged cases, increased in pulmonary pressures are transmitted to the right side of the heart. This can result in a severe form of congestive heart failure known as cor pulmonale. Diastolic function of the heart also becomes affected.[127] Elevated arterial pressure (i.e., hypertension) can be a consequence of OSA syndrome.[128] When hypertension is caused by OSA, it is distinctive in that, unlike most cases (so-called essential hypertension), the readings do not drop significantly when the individual is sleeping (non-dipper) or even increase (inverted dipper).[129]
Without treatment, the sleep deprivation and lack of oxygen caused by sleep apnea increases health risks such as cardiovascular disease, aortic disease (e.g. aortic aneurysm),[130] high blood pressure,[131][132] stroke,[133] diabetes, clinical depression,[134] weight gain, obesity,[33] and even death.
OSA is associated with cognitive impairment, including deficits in inductive and deductive reasoning, attention, vigilance, learning, executive functions, and episodic and working memory. OSA is associated with increased risk for developing mild cognitive impairment and dementia, and has been associated with neuroanatomical changes (reductions in volumes of the hippocampus, and gray matter volume of the frontal and parietal lobes) which can however be at least in part reversed with CPAP treatment.[135][136]
Epidemiology
The prevalence of OSA in the general population is estimated to 27% of middle-aged men and 9% of middle-aged women. However, it is highly underdiagnosed as the OSA is not always accompanied by daytime sleepiness which can leave the sleep-disordered breathing unnoticed.[137] The prevalence of OSA with daytime sleepiness is thus estimated to affect 3% to 7% of men and 2% to 5% of women, and the disease is common in both developed and developing countries.[138] It is most commonly diagnosed in individuals over 65 years old, with estimations ranging from 19% to 57%.[51] The prevalence has drastically increased the past decades mainly explained by the obesity epidemic currently observed.[67]
Men are more affected by OSA than women but, the phenomenology differs between both genders.[33] Snoring and witnessed apnea are more frequent among men but insomnia for example is more frequent among women.[33] The OSA frequency increase with age for the women.[33] The mortality is higher for women.[33]
Some studies report that it is more frequent among the Hispanic and African American population than among white population.[33]
If studied carefully in a sleep lab by polysomnography (formal "sleep study"), it is believed that approximately 1 in 5 American adults would have at least mild OSA.[139]
Research
Neurostimulation is currently being studied as a method of treatment;[140] an implanted hypoglossal nerve stimulation system received European CE Mark (Conformité Européenne) approval in March 2012.[141] Also being studied are exercises of the muscles around the mouth and throat through activities such as playing the didgeridoo.[142][143]
See also
References
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D. (2012). "Rules for the Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events". Journal of Clinical Sleep Medicine. 8 (5): 597–619. doi:10.5664/jcsm.2172. PMC 3459210. PMID 23066376.
- American Academy of Sleep Medicine (2014). International Classification of Sleep Disorders, 3rd edition. Darien, IL: American Academy of Sleep Medicine.
- Tung, P.; Levitzky, Y.S.; Wang, R.; Wenig, J.; Quan, S.F.; Gottlieb, D.J.; Rueschman, M.; Punjabi, N.M.; Bertisch, S.; Benjamin, E.J.; Redline, S. (2017). "Obstructive and Central Sleep Apnea and the Risk of Incident Atrial Fibrillation in a Community Cohort of Men and Women". Journal of the American Heart Association. 6 (7).
- Stoohs, R.A.; Blum, H-C.; Lnaack, L.; Butsch-von-der-Heydt, B.; Guilleminault, C. (2005). "Comparison of Pleural Pressure and Transcutaneous Diaphragmativ Electromyogram in Obstructive Sleep Apnea Syndrome". Sleep. 28 (3): 321–329.
- American Academy of Sleep Medicine. "AASM Practice Guidelines". AASM. Retrieved 19 June 2019.
- Mbata, G.C.; Chukwuka, J.C. (2012). "Obstructive Sleep Apnea-Hypopnea Syndrome". Annals of Medical and Health Sciences Research. 2 (1): 74–79. doi:10.4103/2141-9248.96943. PMC 3507119. PMID 23209996.
- Barnes L, ed. (2009). Surgical pathology of the head and neck (3rd ed.). New York: Informa healthcare. ISBN 9781420091632.:226
- Young, T; Skatrud, J; Peppard, PE (2004). "Risk Factors for Obstructive Sleep Apnea in Adults". JAMA. 291 (16): 2013–2016. doi:10.1001/jama.291.16.2013. PMID 15113821.
- Gale SD, Hopkins RO (2004). "Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea". J Int Neuropsychol Soc. 10 (1): 60–71. doi:10.1017/S1355617704101082. PMID 14751008.
- Henry, D; Rosenthal, L (Feb 2013). ""Listening for his breath:" the significance of gender and partner reporting on the diagnosis, management, and treatment of obstructive sleep apnea". Social Science & Medicine. 79: 48–56. doi:10.1016/j.socscimed.2012.05.021. PMID 22770968.
- Dehlink, Eleonora; Tan, Hui-Leng (2016). "Update on Paediatric Obstructive Sleep Apnea". Journal of Thoracic Disease. 8 (2): 224–235. doi:10.3978/j.issn.2072-1439.2015.12.04. PMC 4739955. PMID 26904263.
- Jazi, SM; Barati, B; Kheradmand, A (2011). "Treatment of adenotonsillar hypertrophy: A prospective randomized trial comparing azithromycin vs. fluticasone". Journal of Research in Medical Sciences. 16 (12): 1590–1597. PMC 3434901. PMID 22973368.
- Capdevila, OS; Kheirandish-Gozal, L; Dayyat, E; Gozal, D (2008). "Pediatric Obstructive Sleep Apnea. Complications, Management, and Long-term Outcomes". Proceedings of the American Thoracic Society. 5 (2): 274–282. doi:10.1513/pats.200708-138MG. PMC 2645258. PMID 18250221.
- Dayyat, E; Kheirandish-Gozal, L; Gozal, D (2007). "Childhood Obstructive Sleep Apnea: One or Two Distinct Disease Entities?". Sleep Medicine Clinics. 2 (3): 433–444. doi:10.1016/j.jsmc.2007.05.004. PMC 2084206. PMID 18769509.
- Kalra, M; Inge, T; Garcia, V; Daniels, S; Lawson, L; Curti, R; Cohen, A; Amin, R (2005). "Obstructive Sleep Apnea in Extremely Overweight Adolescents undergoing Bariatric Surgery". Obesity Research. 13 (7): 1175–1179. doi:10.1038/oby.2005.139. PMID 16076986.
- Verhulst, SL; Franckx, H; Van Gaal, L; De Backer, W; Desager, K (2009). "The Effect of Weight Loss on Sleep-Disordered Breating in Obese Teenagers". Obesity (Silver Spring). 17 (6): 1178–1183. doi:10.1038/oby.2008.673. PMID 19265797.
- Bower, CM; Gungor, A (2000). "Pediatric Obstructive Sleep Apnea Syndrome". Otolaryngologic Clinics of North America. 33 (1): 49–75. doi:10.1016/s0030-6665(05)70207-3. PMID 10637344.
- Halbower AC, Degaonkar M, Barker PB, et al. (August 2006). "Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury". PLoS Med. 3 (8): e301. doi:10.1371/journal.pmed.0030301. PMC 1551912. PMID 16933960.
- Schwab, Richard J.; Kim, Christopher; Bagchi, Sheila; Keenan, Brendan T.; Comyn, François-Louis; Wang, Stephen; Tapia, Ignacio E.; Huang, Shirley; Traylor, Joel; Torigian, Drew A.; Bradford, Ruth M.; Marcus, Carole L. (2015). "Understanding the Anatomic Basis for Obstructive Sleep Apnea Syndrome in Adolescents". American Journal of Respiratory and Critical Care Medicine. 191 (11): 1295–1309. doi:10.1164/rccm.201501-0169OC. PMC 4476519. PMID 25835282.
- Ezzedini R, Darabi M, Ghasemi B, Darabi M, Fayezi S, Moghaddam YJ, et al. (2013). "Tissue fatty acid composition in obstructive sleep apnea and recurrent tonsillitis". Int J Pediatr Otorhinolaryngol. 77 (6): 1008–12. doi:10.1016/j.ijporl.2013.03.033. PMID 23643333.
- Edwards, Natalie; Sullivan, Colin E. (2008). "Sleep-Disordered Breathing in Pregnancy". Sleep Medicine Clinics. 3: 81–95. doi:10.1016/j.jsmc.2007.10.010.
- Dayyat, E.; Kheirandish-Gozal, L.; Gozal, D. (2008). "Childhood Obstructive Sleep Apnea: One or Two Distinct Disease Entities?". Sleep Medicine Clinics. 2 (3): 433–444. doi:10.1016/j.jsmc.2007.05.004. PMC 2084206. PMID 18769509.
- "Sleep Apnea: Risk Factors". Mayo Clinic. June 29, 2010. Archived from the original on 2011-12-17. Retrieved November 4, 2010.
- Lu, LR; Peat, JK; Sullivan, CE (2003). "Snoring in Preschool Children: Prevalence and Association with Nocturnal Cough and Asthma". Chest. 124 (2): 587–593. doi:10.1378/chest.124.2.587. PMID 12907547.
- McColley, SA; Carroll, JL; Curtis, S; Loughlin, GM; Sampson, HA (1997). "High Prevalence of Allergic Sensitization in Children with Habitual Snoring and Obstructive Sleep Apnea". Chest. 111 (1): 170–173. doi:10.1378/chest.111.1.170. PMID 8996012.
- de Miguel-Díez J, Villa-Asensi JR, Alvarez-Sala JL (December 2003). "Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children". Sleep. 26 (8): 1006–9. doi:10.1093/sleep/26.8.1006. PMID 14746382.
- Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R (April 2006). "Obstructive sleep apnea: Should all children with Down syndrome be tested?". Arch. Otolaryngol. Head Neck Surg. 132 (4): 432–6. doi:10.1001/archotol.132.4.432. PMID 16618913.
- Sloan GM (March 2000). "Posterior pharyngeal flap and sphincter pharyngoplasty: the state of the art". Cleft Palate Craniofac. J. 37 (2): 112–22. doi:10.1597/1545-1569(2000)037<0112:PPFASP>2.3.CO;2. PMID 10749049.
- Pugh, M.B. et al. (2000). Apnea. Stedman's Medical Dictionary (27th ed.) Retrieved June 18, 2006 from STAT!Ref Online Medical Library database.
- Liao YF, Noordhoff MS, Huang CS, et al. (March 2004). "Comparison of obstructive sleep apnea syndrome in children with cleft palate following Furlow palatoplasty or pharyngeal flap for velopharyngeal insufficiency". Cleft Palate Craniofac. J. 41 (2): 152–6. doi:10.1597/02-162. PMID 14989690.
- McWilliams, Betty Jane; Peterson-Falzone, Sally J.; Hardin-Jones, Mary A.; Karnell, Michael P. (2001). Cleft palate speech (3rd ed.). St. Louis: Mosby. ISBN 978-0-8151-3153-3.
- Gross, JB; Bachenberg, KL; Benumof, JL; Caplan, RA; Connis, RT; Coté, CJ; Nickinovich, DG; Prachand, V; Ward, DS; Weaver, EM; Ydens, L; Yu, S; American Society of Anesthesiologists Task Force on Perioperative Management (May 2006). "Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea". Anesthesiology. 104 (5): 1081–93, quiz 1117–8. doi:10.1097/00000542-200605000-00026. PMID 16645462.
- Foldvary-Schaefer, N. R., & Waters, T. E. (2017). Sleep-Disordered Breathing: CONTINUUM: Lifelong Learning in Neurology, 23(4), 1093‑1116. https://doi.org/10.1212/01.CON.0000522245.13784.f6
- Walter, L.M.; Horne, R.S.C. (2018). "Obstructive Sleep-Disordered Breathing in Children; impact on the Developing Brain". Pediatric Respirology and Critical Care Medicine. 2 (4): 58–64. doi:10.4103/prcm.prcm_16_18.
- Chervin, R.D.; Archbold, K.H. (2001). "Hyperactivity and Polysomnographic Findings in Children Evaluated for Sleep-Disordered Breathing". Sleep. 24 (3): 313–320. doi:10.1093/sleep/24.3.313. PMID 11322714.
- Capdevila, Oscar Sana; Kheirandish-Gozal, Leila; Dayyat, Ehab; Gozal, David (2008). "Pediatric Obstructive Sleep Apnea. Complications, Management, and Long-term Outcomes". Proceedings of the American Thoracic Society. 5 (2): 274–282. doi:10.1513/pats.200708-138MG. PMC 2645258. PMID 18250221.
- O'Brien, L.M.; Mervis, C.B.; Holbrook, C.R.; Bruner, J.L.; Smith, N.H.; McNally, N.; McClimment, M.C.; Gozal, D (2004). "Neurobehavioural Correlates of Sleep-Disordered Breathing in Children". Journal of Sleep Research. 13 (2): 165–172. doi:10.1111/j.1365-2869.2004.00395.x. PMID 15175097.
- Goyal, A.; Pakhare, A.P.; Bhatt, G.C.; Choudhary, B.; Patil, R. (2018). "Association of Pediatric Obstructive Sleep Apnea with Poor Academic Performance: A School-Based Study from India". Lung India. 35 (2): 132–136. doi:10.4103/lungindia.lungindia_218_17 (inactive 2019-10-04). PMID 29487248.
- Galland, B; Spruyt, K; Dawes, P; McDowall, PS; Elder, D; Schaughency, E (2015). "Sleep Disordered Breathing and Academic Performance: A Meta-Analysis". Pediatrics. 136 (4): e934–e946. doi:10.1542/peds.2015-1677. PMID 26347434.
- Amin, R; Somers, VK; McConnell, K; Willging, P; Myer, C; Sherman, M; McPhail, G; Morgenthal, A; Fenchel, M; Bean, J; Kimball, T; Daniels, S (2008). "Activity-Adjusted 24-Hour Ambulatory Blood Pressure and Cardiac Remodeling in Children with Sleep Disordered Breathing". Hypertension. 51: 84–91. doi:10.1161/HYPERTENSIONAHA.107.099762. PMID 18071053.
- Ip, MS; Lam, B; Ng, MM (2002). "Obstructive Sleep Apnea is Indenpendently Associated with Insulin Resistance". American Journal of Respiratory and Critical Care Medicine. 165 (5): 670–676. doi:10.1164/ajrccm.165.5.2103001. PMID 11874812.
- Basha, S; Bialowas, C; Ende, K; Szeremeta, W (2009). "Effectiveness of Adenotonsillectomy in the Resolution of Nocturnal Enuresis Secondary to Obstructive Sleep Apnea". The Laryngoscope. 115 (6): 1101–1103. doi:10.1097/01.MLG.0000163762.13870.83. PMID 15933530.
- Barone, JG; Hanson, C; DaJusta, DG; Gioia, K; England, SJ; Schneider, D (2009). "Nocturnal Enuresis and Overweight are Associated with Obstructive Sleep Apnea". Pediatrics. 124 (1).
- Brooks, LJ; Tropol, HI (2003). "Enuresis in Children with Sleep Apnea". The Journal of Pediatrics. 142 (5): 515–518. doi:10.1067/mpd.2003.158. PMID 12756383.
- Weissbach, A; Leiberman, A; Tarasiuk, A; Goldbart, A; Tal, A (2006). "Adenotonsillectomy improves Enuresis in Children with Obstructive Sleep Apnea Syndrome". International Journal of Pediatric Otorhinolaryngology. 70 (8): 1351–1356. doi:10.1016/j.ijporl.2006.01.011. PMID 16504310.
- Carotenuto, M; Esposito, M; Parisi, L; Gallai, B; Marotta, R; Pascotto, A; Roccella, M (2012). "Depressive Symptoms and Childhood Sleep Apnea Syndrome". Neuropsychiatric Disease and Treatment. 8: 369–373. doi:10.2147/NDT.S35974. PMC 3430390. PMID 22977304.
- Yilmaz, E; Sedky, K; Bennet, DS (2013). "The Relationship between Depressive Symptoms and Obstructive Sleep Apnea in Pediatric Populations: A Meta-Analysis". Journal of Clinical Sleep Medicine. 9 (11): 1213–20. doi:10.5664/jcsm.3178. PMC 3805811. PMID 24235907.
- Crabtree, VM; Varni, JW; Gozal, D (2004). "Health-Related Quality of Life and Depressive Symptoms in Children with Suspected Sleep-Disordered Breathing". Sleep. 27 (6): 1131–1138. doi:10.1093/sleep/27.6.1131. PMID 15532207.
- Alsubie, HS; BaHammam, AS (2017). "Obstructive Sleep Apnoea: Children are not Little Adults". Paediatric Respiratory Reviews. 21: 72–79. doi:10.1016/j.prrv.2016.02.003. PMID 27262609.
- McNicholas, WT (2008). "Diagnosis of Obstructive Sleep Apnea in Adults". Proceedings of the American Thoracic Society. 5 (2).
- Stranks, Elizabeth K.; Crowe, Simon F (2016). "The Cognitive Effects of Obstructive Sleep Apnea: An Update Meta-Analysis". Archives of Clinical Neuropsychology. 31 (2): 186–193. doi:10.1093/arclin/acv087. PMID 26743325.
- Robichaud-Hallé, L; Beaudry, M; Fortin, M (2012). "Obstructive Sleep Apnea and Multimorbidity". BMC Pulmonary Medicine. 12 (60): 60. doi:10.1186/1471-2466-12-60. PMC 3515504. PMID 23006602.
- Romero-Corral, A; Caples, SM; Lopez-Jimenez, F; Somers, VK (2010). "Interactions Between Obesity and Obstructive Sleep Apnea". Chest. 137 (3): 711–719. doi:10.1378/chest.09-0360. PMC 3021364. PMID 20202954.
- Fulda, S; Schulz, H (2003). "Cognitive Dysfunction in Sleep-Related Breathing Disorders: A Meta-Analysis". Sleep Research Online. 5 (1): 19–51.
- Bucks, RS; Olaithe, M; Eastwood, P (2012). "Neurocognitive Function in Obstructuve Sleep Apnea: A Meta-Review". Respirology. 18 (1): 61–70. doi:10.1111/j.1440-1843.2012.02255.x. PMID 22913604.
- Nejati, V; Salehinejad, MA; Nitsche, MA (2018). "Interaction of the Left Dorsolateral Prefrontal Cortex (l-DLPFC) and Right Orbitofrontal Cortex (OFC) in Hot and Cold Executive Functions: Evidence from Transcranial Direct Current Stimulation (tDCS)". Neuroscience. 369: 109–123. doi:10.1016/j.neuroscience.2017.10.042. PMID 29113929.
- Beebe, DW; Groesz, L; Wells, C; Nichols, A; McGee, K (2003). "The Neuropsychological Effects of Obstructive Sleep Apnea: A Meta-Analysis of Norm-Referenced and Case-Controlled Data". Sleep. 26 (3): 298–307. doi:10.1093/sleep/26.3.298. PMID 12749549.
- Wallace, A; Bucks, RS (2013). "Memory and Obstructive Sleep Apnea: A Meta-Analysis". Sleep. 36 (2): 203–220. doi:10.5665/sleep.2374. PMC 3543053. PMID 23372268.
- Olaithe, M; Bucks, RS; Hillman, DR; Eastwood, PR (2017). "Cognitive Deficits in Obstructive Sleep Apnea: Insights from a Meta-Review and Comparison with Deficits Observed in COPD, Insomnia and Sleep Deprivation". Sleep Medicine Reviews. 38: 39–49. doi:10.1016/j.smrv.2017.03.005. PMID 28760549.
- Cori, JM; Jackson, ML; Barnes, M; Westlake, J; Emerson, P; Lee, J; Galante, R; Hayley, A; Wilsmore, N; Kennedy, GA; Howard, M (2018). "The Differential Effects of Regular Shift Work and Obstructive Sleep Apnea on Sleepiness, Mood and Neurocognitive Function". Journal of Clinical Sleep Medicine. 14 (6): 941–951. doi:10.5664/jcsm.7156. PMC 5991942. PMID 29852909.
- Slater, G; Steier, J (2012). "Excessive Daytime Sleepiness in Sleep Disorders". Journal of Thoracic Disease. 4 (6): 608–616. doi:10.3978/j.issn.2072-1439.2012.10.07. PMC 3506799. PMID 23205286.
- LaGrotte, C; Fernandez-Mendoza, J; Calhoun, SL; Liao, D; Bixler, EO; Vgontzas, AN (2016). "The relative Association of Obstructive Sleep Apnea, Obesity and Excessive Daytime Sleepiness with Incident Depression: A Longitudinal, Population-Based Study". International Journal of Obesity. 40 (9): 1397–1404. doi:10.1038/ijo.2016.87. PMC 5014694. PMID 27143032.
- Mulgrew, AT; Ryan, CF; Fleetham, JA; Cheema, R; Fox, N; Koehoorn, M; Fitzgerald, JM; Marra, C; Ayas, NT (2007). "The Impact of Obstructive Sleep Apnea and Daytime Sleepiness on Work Limitation". Sleep Medicine. 9 (1): 42–53. doi:10.1016/j.sleep.2007.01.009. PMID 17825611.
- Guilleminault, C; Brooks, SN (2001). "Excessive Daytime Sleepiness: A Challenge for the Practising Neurologist". Brain. 124 (8): 1482–1491. doi:10.1093/brain/124.8.1482. PMID 11459741.
- Jackson, ML; McEvoy, RD; Banks, S; Barnes, M (2018). "Neurobehavioral Impairment and CPAP Treatment Response in Mild-Moderate Obstructive Sleep Apneas". Journal of Clinical Sleep Medicine. 14 (1): 47–56. doi:10.5664/jcsm.6878. PMC 5734892. PMID 29198304.
- Eskandari, D; Zou, D; Grote, L; Schneider, H; Penzel, T; Hedner, J (2017). "Independent Associations between Arterial Bicarbonate, Apnea Severity and Hypertension in Obstructive Sleep Apnea". Respiratory Research. 18 (130): 130. doi:10.1186/s12931-017-0607-9. PMC 5490198. PMID 28659192.
- Drager, LF; Togeiro, SM; Polotsky, VY; Lorenzi-Filho, G (2013). "Obstructive Sleep Apnea: A CardioMetabolic Risk in Obesity and Metabolic Syndrome". Journal of the American College of Cardiology. 62 (7): 569–576. doi:10.1016/j.jacc.2013.05.045. PMC 4461232. PMID 23770180.
- Ashrafian, H; Toma, T; Rowland, SP; Harling, L; Tan, A; Efthimiou, E; Darzi, A; Athanasiou, T (2015). "Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses". Obesity Surgery. 25 (7): 1239–1250. doi:10.1007/s11695-014-1533-2. hdl:10044/1/54731. PMID 25537297.
- Sharma, SK; Agrawal, S; Damodaran, D; Sreenivas, V; Kadhiravan, T; Lakshmy, R; Jagia, P; Kumar, A (2011). "CPAP for the Metabolic Syndrome in Patients with Obstructive Sleep Apnea". The New England Journal of Medicine. 365 (24): 2277–2286. doi:10.1056/NEJMoa1103944. PMID 22168642.
- Dobrosielski, DA; Patil, S; Schwartz, AR; Bandeen-Roche, K; Stewart, KJ (2015). "Effects of Exercise and Weight Loss in Older Adults with Obstructive Sleep Apnea". Medicine and Science in Sports and Exercise. 47 (1): 20–26. doi:10.1249/MSS.0000000000000387. PMC 4246024. PMID 24870569.
- Guglielmi, O; Magnavita, N; Garbarino, S (2018). "Sleep Quality, Obstructive Sleep Apnea, and Psychological Distress in Truck Drivers: A Cross-Sectional Study". Social Psychiatry and Psychiatric Epidemiology. 53 (5): 531–536. doi:10.1007/s00127-017-1474-x. PMID 29285594.
- Olaithe, M; Nanthakumar, S; Eastwood, PR; Bucks, RS (2015). "Cognitive and Mood Dysfunction in Adult Obstructive Sleep Apnoea (OSA): Implications for Psychological Research and Practice". Translational Issues in Psychological Science. 1 (1): 67–78. doi:10.1037/tps0000021.
- Garbarino, S; Bardwell, WA; Guglielmi, O; Chiorri, C; Bonanni, E; Magnavita, N (2018). "Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis". Behavioural Sleep Medicine. 1 (23): 1–23. doi:10.1080/15402002.2018.1545649. PMID 30453780.
- Xiao, Y; Han, D; Zang, H; Wang, D (2016). "The effectiveness of nasal surgery on psychological symptoms in patients with obstructive sleep apnea and nasal obstruction". Acta Oto-Laryngologica. 136 (6): 626–632. doi:10.3109/00016489.2016.1143120. PMID 26903174.
- Gupta, MA; Simpson, FC (2015). "Obstructive Sleep Apnea and Psychiatric Disorders: A Systemic Review". Journal of Clinical Sleep Medicine. 11 (2): 165–175. doi:10.5664/jcsm.4466. PMID 25406268.
- Jonas, DE; Amick, HR; Feltner, C; Weber, RP; Arvanitis, M; Stine, A; Lux, L; Harris, RP (2017). "Screening for Obstructive Sleep Apnea in Adults. Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 317 (4): 415–433. doi:10.1001/jama.2016.19635. PMID 28118460.
- Charokopos, A; Card, ME; Gunderson, C; Steffens, C; Bastian, LA (2018). "The Association of Obstructive Sleep Apnea and Pain Outcomes in Adults: A Systematic Review". Pain Medicine. 19: 69–75. doi:10.1093/pm/pny140. PMID 30203008.
- "Sleep Health & Wellness Blog". Sleep Education. American Academy of Sleep Medicine (AASM). Retrieved 2019-06-13.
- Fietze I, Penzel T, Alonderis A, et al. (February 2011). "Management of obstructive sleep apnea in Europe". Sleep Med. 12 (2): 190–7. doi:10.1016/j.sleep.2010.10.003. PMID 21167776.
- Jonas, Daniel E; Amick, Halle R; Feltner, Cynthia; Weber, Rachel P; Arvanitis, Marina; Stine, A; Lux, L; Harris, Russell P (2017). "Screening for Obstructive Sleep Apnea in Adults Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 317 (4): 415–433. doi:10.1001/jama.2016.19635. PMID 28118460.
- US Preventive Services Task Force (2017). "Screening for Obstructive Sleep Apnea in Adults US Preventive Services Task Force Recommendation Statement". JAMA. 317 (4): 407–414. doi:10.1001/jama.2016.20325. PMID 28118461.
- Franklin, Karl A.; Lindberg, Eva (2015). "Obstructive Sleep Apnea is a common disorder in the population - a review on the epidemiology of Sleep Apnea". Journal of Thoracic Disease. 7 (8): 1311–1322. doi:10.3978/j.issn.2072-1439.2015.06.11. PMC 4561280. PMID 26380759.
- "Sleep-related Breathing Disorders in Adults: recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. The Report of an American Academy of Sleep Medicine Task Force". Sleep. 22 (5): 667–689. 1999. doi:10.1093/sleep/22.5.667.
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R. (2017). "Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline". Journal of Clinical Sleep Medicine. 13 (3): 479–504. doi:10.5664/jcsm.6506. PMID 28162150.
- Thurnheer, Robert (2007). "Diagnosis of the Obstructive Sleep Apnoea Syndrome" (PDF). European Respiratory Disease.
- Crook, S.; Sievi, N.A.; Bloch, K.E.; Stradling, J.R.; Frei, A.; Puhan, M.A.; Kohler, M. (2019). "Minimum important difference of the Epworth Sleepiness Scale in Obstructive Sleep Apnoea: estimation from three randomised controlled trials". Thorax. 74 (4): 390–396. doi:10.1136/thoraxjnl-2018-211959 (inactive 2019-10-04). PMID 30100576.
- Chiu, HY; Chen, PY; Chuang, LP; Chen, NH; Tu, YK; Hsieh, YJ; Wang, YC; Guilleminault, C (2017). "Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis". Sleep Medicine Reviews. 36: 57–70. doi:10.1016/j.smrv.2016.10.004. PMID 27919588.
- Amra, B; Javani, M; Soltaninejad, F; Penzel, T; Fietze, I; Schoebel, C; Farajzadegan, Z (2018). "Comparison of Berlin Questionnaire, STOP-Bang, and Epworth Sleepiness Scale for Diagnosing Obstructive Sleep Apnea in Persian Patients". International Journal of Preventive Medicine. 9 (28): 28. doi:10.4103/ijpvm.IJPVM_131_17. PMC 5869953. PMID 29619152.
- American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014.
- "Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force". Sleep. 22 (5): 667–89. August 1999. doi:10.1093/sleep/22.5.667. PMID 10450601.
- Mulgrew AT, Fox N, Ayas NT, Ryan CF (February 2007). "Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study". Annals of Internal Medicine. 146 (3): 157–66. doi:10.7326/0003-4819-146-3-200702060-00004. PMID 17283346.
- Flemons WW, Whitelaw WA, Brant R, Remmers JE (November 1994). "Likelihood ratios for a sleep apnea clinical prediction rule". Am. J. Respir. Crit. Care Med. 150 (5 Pt 1): 1279–85. doi:10.1164/ajrccm.150.5.7952553. PMID 7952553.
- Friedman: Sleep Apnea and Snoring, 1st ed. 2008
- Azagra-Calero, E; Espinar-Escalona, E; Barrera-Mora, JM; Llamas-Carreras, JM; Solano-Reina, E (Nov 1, 2012). "Obstructive sleep apnea syndrome (OSAS). Review of the literature". Medicina Oral, Patologia Oral y Cirugia Bucal. 17 (6): e925–9. doi:10.4317/medoral.17706. PMC 3505711. PMID 22549673.
- Qaseem, A; Holty, JE; Owens, DK; Dallas, P; Starkey, M; Shekelle, P; for the Clinical Guidelines Committee of the American College of, Physicians (Sep 24, 2013). "Management of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 159 (7): 471–83. doi:10.7326/0003-4819-159-7-201310010-00704. PMID 24061345.
- Bratton, DJ; Gaisl, T; Wons, AM; Kohler, M (1 December 2015). "CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis". JAMA. 314 (21): 2280–93. doi:10.1001/jama.2015.16303. PMID 26624827.
- Iftikhar, IH; Kline, CE; Youngstedt, SD (Sep 29, 2013). "Effects of Exercise Training on Sleep Apnea: A Meta-analysis". Lung. 192 (1): 175–184. doi:10.1007/s00408-013-9511-3. PMC 4216726. PMID 24077936.
- Giles, TL; Lasserson, TJ; Smith, BH; White, J; Wright, J; Cates, CJ (Jul 19, 2006). "Continuous positive airways pressure for obstructive sleep apnoea in adults". The Cochrane Database of Systematic Reviews (3): CD001106. doi:10.1002/14651858.CD001106.pub3. PMID 16855960.
- Riaz, M; Certal, V; Nigam, G; Abdullatif, J; Zaghi, S; Kushida, CA; Camacho, M (2015). "Nasal Expiratory Positive Airway Pressure Devices (Provent) for OSA: A Systematic Review and Meta-Analysis". Sleep Disorders. 2015: 734798. doi:10.1155/2015/734798. PMC 4699057. PMID 26798519.
- Whitelaw WA, Brant RF, Flemons WW (January 2005). "Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea". Am. J. Respir. Crit. Care Med. 171 (2): 188–93. doi:10.1164/rccm.200310-1360OC. PMID 15486338. Review in: Caples SM (2005). "The accuracy of physicians in predicting successful treatment response in suspected obstructive sleep apnea did not differ between home monitoring and polysomnography". ACP J. Club. 143 (1): 21. PMID 15989309.
- Littner M, Hirshkowitz M, Davila D, et al. (March 2002). "Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine report". Sleep. 25 (2): 143–7. doi:10.1093/sleep/25.2.143. PMID 11902424.
- McNicholas, Walter T.; Bonsignore, Maria R.; Lévy, Patrick; Ryan, Silke (2016-05-27). "Mild obstructive sleep apnoea: clinical relevance and approaches to management". The Lancet Respiratory Medicine. 4 (10): 826–834. doi:10.1016/S2213-2600(16)30146-1. ISSN 2213-2619. PMID 27245915.
- Neill AM, Angus SM, Sajkov D, McEvoy RD (January 1997). "Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea". Am. J. Respir. Crit. Care Med. 155 (1): 199–204. doi:10.1164/ajrccm.155.1.9001312. PMID 9001312.
- Nakano H, Ikeda T, Hayashi M, Ohshima E, Onizuka A (March 2003). "Effects of body position on snoring in apneic and nonapneic snorers". Sleep. 26 (2): 169–72. doi:10.1093/sleep/26.2.169. PMID 12683476.
- Loord H, Hultcrantz E (August 2007). "Positioner--a method for preventing sleep apnea". Acta Otolaryngol. 127 (8): 861–8. doi:10.1080/00016480601089390. PMID 17762999.
- Szollosi I, Roebuck T, Thompson B, Naughton MT (August 2006). "Lateral sleeping position reduces severity of central sleep apnea / Cheyne-Stokes respiration". Sleep. 29 (8): 1045–51. doi:10.1093/sleep/29.8.1045. PMID 16944673.
- "Learning To Play A Wind Instrument Could Lower Your Risk For Obstructive Sleep Apnea". medicaldaily.com. 16 April 2015. Archived from the original on 18 March 2018. Retrieved 24 April 2018.
- Ward CP, York KM, McCoy JG (2012). "Risk of obstructive sleep apnea lower in double reed wind musicians". J Clin Sleep Med. 8 (3): 251–5. doi:10.5664/jcsm.1906. PMC 3365082. PMID 22701381.
- "Submucosal Ablation of the Tongue Base for OSAS". American Academy of Otolaryngology–Head and Neck Surgery. Archived from the original on 17 October 2013. Retrieved 29 October 2013.
- "Sleep apnea". University of Maryland Medical Center. Archived from the original on 2008-04-30.
- "Premarket Approval (PMA)". Archived from the original on 2014-05-06. Retrieved 2014-05-05.. FDA "Premarket Approval (PMA) Inspire II Upper Airway Stimulation System" U.S. Food and Drug Administration. April 30, 2014.
- Strollo PJ Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, et al. (STAR Trial Group) (January 9, 2014). "Upper-airway stimulation for obstructive sleep apnea". New England Journal of Medicine. 370 (2): 139–49. doi:10.1056/nejmoa1308659. PMID 24401051.
- Farrar, J; Ryan; Olliver; Gillespie (October 2008). "Radiofrequency ablation for the treatment of obstructive sleep apnea: a meta-analysis". The Laryngoscope. 118 (10): 1878–83. doi:10.1097/MLG.0b013e31817d9cc1. PMID 18806478.
- Eick, Olaf J (1 July 2002). "Temperature Controlled Radiofrequency Ablation". Indian Pacing Electrophysiol. 3. 2 (3): 66–73. PMC 1564057. PMID 17006561.
- Bashetty, Kusum; Gururaj Nadig Sandhya Kapoor (19 November 2009). "Electrosurgery in aesthetic and restorative dentistry: A literature review and case reports". Journal of Conservative Dentistry. 12 (4): 139–144. doi:10.4103/0972-0707.58332. PMC 2879725. PMID 20543922.
- Weaver, TE; Grunstein, RR (2008). "Adherence to Continuous Positive Airway Pressure Therapy. The Challenge to Effective Treatment". Proceedings of the American Thoracic Society. 5 (2).
- Steward, DL; Weaver, Woodson (2005). "Multilevel temperature-controlled radiofrequency for obstructive sleep apnea: extended follow-up". Otolaryngol Head Neck Surg. 132 (4): 630–5. doi:10.1016/j.otohns.2004.11.013. PMID 15806059.
- Gaisl, Thomas; Haile, Sarah R.; Thiel, Sira; Osswald, Martin; Kohler, Malcolm (August 2019). "Efficacy of pharmacotherapy for OSA in adults: A systematic review and network meta-analysis". Sleep Medicine Reviews. 46: 74–86. doi:10.1016/j.smrv.2019.04.009. ISSN 1532-2955. PMID 31075665.
- Veasey SC (2003). "Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential". Am J Respir Med. 2 (1): 21–9. doi:10.1007/BF03256636. PMID 14720019.
- Babson, Kimberly; Sottile, James; Morabito, Danielle (2017). "Cannabis, Cannabinoids, and Sleep: a Review of the Literature". Current Psychiatry Reports. 19 (23): 23. doi:10.1007/s11920-017-0775-9. PMID 28349316.
- Carley, David W.; Prasad, Bharati; Reid, Kathryn J; Malkani, Roneil; Attarian, Hryar; Abbott, Sabra M; Vern, B; Xie, Hui; Yuan, Chengbo; Zee, Phyllis (2018). "Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea". Sleep. 41 (1). doi:10.1093/sleep/zsx184. PMC 5806568. PMID 29121334.
- Ramar, Kannan; Rosen, Ilene M; Kirsch, Douglas B; Chervin, Ronald D.; Carden, Kelly A. (2018). "Medical Cannabis and The Treatment of Obstructive Sleep Apnea: An American Academy of Sleep Medicine Position Statement". Journal of Clinical Sleep Medicine. 14 (4): 679–681. doi:10.5664/jcsm.7070. PMC 5886446. PMID 29609727.
- Kolla, BP; Mansukhani, MP; Olson, EJ; St. Louis, EK; Silber, MH; Morgenthaler, TI (2018). "Medical Cannabis for Obstructive Sleep Apnea: Premature and Potentially Harmful". Mayo Clinic Proceedings. 93 (6): 689–692. doi:10.1016/j.mayocp.2018.04.011. PMID 29866280.
- Ramar, Kannan; Kirsch, Douglas B.; Carden, Kelly A.; Rosen, Ilene M.; Malhotra, Raman K. (2018). "Medical Cannabis, Synthetic Marijuana Extracts and Obstructive Sleep Apnea". Journal of Clinical Sleep Medicine. 14 (10): 1815–1816. doi:10.5664/jcsm.7412. PMC 6175803. PMID 30353829.
- Franklin, Karl A.; Lindberg, Eva (2015-07-27). "Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea". Journal of Thoracic Disease. 7 (8): 1311–1322. doi:10.3978/j.issn.2072-1439.2015.06.11. PMC 4561280. PMID 26380759.
- N.A. Shah, N.A. Botros, H.K. Yaggi, V. Mohsenin (May 20, 2007). "Sleep Apnea Increases Risk of Heart Attack or Death by 30%" (Press release). New Haven, Connecticut: American Thoracic Society. Archived from the original on May 24, 2009.CS1 maint: uses authors parameter (link)
- Sun Y (Apr 2014). "Cardiac structural and functional changes in old elderly patients with obstructive sleep apnoea-hypopnoea syndrome". J Int Med Res. 42 (2): 395–404. doi:10.1177/0300060513502890. PMID 24445697.
- Silverberg DS, Iaina A, Oksenberg A (January 2002). "Treating obstructive sleep apnea improves essential hypertension and quality of life". Am Fam Physician. 65 (2): 229–36. PMID 11820487.
- Grigg-Damberger M (February 2006). "Why a polysomnogram should become part of the diagnostic evaluation of stroke and transient ischemic attack". J Clin Neurophysiol. 23 (1): 21–38. doi:10.1097/01.wnp.0000201077.44102.80. PMID 16514349.
- Gaisl, Thomas; Bratton, Daniel J.; Kohler, Malcolm (2015-08-01). "The impact of obstructive sleep apnoea on the aorta". The European Respiratory Journal. 46 (2): 532–544. doi:10.1183/09031936.00029315. ISSN 1399-3003. PMID 26113685.
- Paul E. Peppard, Terry Young, Mari Palta, and James Skatrud (May 11, 2000). "Prospective Study of the Association Between Sleep-Disordered Breathing and Hypertension" (PDF). The New England Journal of Medicine. 342 (19): 1378–1384. doi:10.1056/nejm200005113421901. PMID 10805822. Archived (PDF) from the original on September 24, 2015. Retrieved 2013-05-29.
We found a dose–response association between sleep-disordered breathing at base line and the presence of hypertension four years later that was independent of known confounding factors. The findings suggest that sleep-disordered breathing is likely to be a risk factor for hypertension and consequent cardiovascular morbidity in the general population.
CS1 maint: uses authors parameter (link) - Peretz Lavie, Paula Herer, Victor Hoffstein (19 February 2000). "Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study" (PDF). BMJ. 320 (7233): 479–82. doi:10.1136/bmj.320.7233.479. PMC 27290. PMID 10678860. Archived (PDF) from the original on 24 September 2015. Retrieved 2013-05-29.
Conclusion: Sleep apnoea syndrome is profoundly associated with hypertension independent of all relevant risk factors.
CS1 maint: uses authors parameter (link) - Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (November 2005). "Obstructive sleep apnea as a risk factor for stroke and death". N. Engl. J. Med. 353 (19): 2034–41. doi:10.1056/NEJMoa043104. PMID 16282178.
- Schröder CM, O'Hara R (June 2005). "Depression and Obstructive Sleep Apnea (OSA)". Ann Gen Psychiatry. 4: 13. doi:10.1186/1744-859X-4-13. PMC 1181621. PMID 15982424.
- Gagnon, K.; Baril, A.-A.; Gagnon, J.-F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. (October 2014). "Cognitive impairment in obstructive sleep apnea". Pathologie Biologie. 62 (5): 233–240. doi:10.1016/j.patbio.2014.05.015. PMID 25070768.
- Lal, Chitra; Strange, Charlie; Bachman, David (June 2012). "Neurocognitive impairment in obstructive sleep apnea". Chest. 141 (6): 1601–1610. doi:10.1378/chest.11-2214. ISSN 1931-3543. PMID 22670023.
- Olaithe, Michelle; Nanthakumar, Shenooka; Eastwood, Peter R.; Bucks, Romola S. (2015). "Cognitive and Mood Dysfunction in Adult Obstructive Sleep Apnea (OSA): Implications for Psychological Research and Practice". Translational Issues in Psychological Science. 1 (1): 67–78. doi:10.1037/tps0000021.
- Punjabi, N. M. (15 February 2008). "The Epidemiology of Adult Obstructive Sleep Apnea". Proceedings of the American Thoracic Society. 5 (2): 136–143. doi:10.1513/pats.200709-155MG. PMC 2645248. PMID 18250205.
- Shamsuzzaman AS, Gersh BJ, Somers VK (October 2003). "Obstructive sleep apnea: implications for cardiac and vascular disease". JAMA. 290 (14): 1906–14. doi:10.1001/jama.290.14.1906. PMID 14532320.
- Kezirian, EJ; Boudewyns, A; Eisele, DW; Schwartz, AR; Smith, PL; Van de Heyning, PH; De Backer, WA (October 2010). "Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea". Sleep Medicine Reviews. 14 (5): 299–305. doi:10.1016/j.smrv.2009.10.009. PMID 20116305.
- Editors (15 March 2012). "ImThera aura6000 System for Sleep Apnea Gets a Go in Europe". Medgadget. Archived from the original on 16 January 2014. Retrieved 15 January 2014.
- Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O (February 2006). "Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial". BMJ. 332 (7536): 266–70. doi:10.1136/bmj.38705.470590.55. PMC 1360393. PMID 16377643.
- Guimarães KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G (May 2009). "Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome". Am. J. Respir. Crit. Care Med. 179 (10): 962–6. doi:10.1164/rccm.200806-981OC. PMID 19234106.