Brentuximab vedotin
Brentuximab vedotin (INN, trade name Adcetris) is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL). It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL (a type of T cell non-Hodgkin lymphoma).[1]
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric (mouse/human) |
| Target | CD30 |
| Clinical data | |
| Trade names | Adcetris |
| Other names | SGN-35, previously cAC10-vcMMAE |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C6476H9930N1690O2030S40 (C68H105N11O15)3–5 |
| Molar mass | 149.2–151.8 kg/mol |
| | |
Approvals and indications
In August 2011, the U.S. FDA granted accelerated approval to the biologics license application (BLA) submitted by Seattle Genetics for the use of brentuximab vedotin[2] in the treatment of relapsed HL and ALCL.[3] In March 2018, the FDA granted an expansion of use to include first-line treatment of stage III and IV HL in combination with chemotherapy.[4]
In October 2012, the European Medicines Agency (EMA) gave it conditional marketing authorization for relapsed or refractory HL and ALCL.[5]
In November 2017, the FDA approved brentuximab vedotin as a treatment for patients with cutaneous T-cell lymphoma (CTCL) who have received prior systemic therapy.[6] This approval is for patients with primary cutaneous anaplastic large cell lymphoma (pcALCL) and CD30-expressing mycosis fungoides (MF).[6]
Approval In Australia
The Australian PBAC (Pharmaceutical Benefits Advisory Committee) considered a March 2014 application by the manufacturer for inclusion of Brentuximab Vedotin under a Pharmaceutical Benefits Scheme Section 100 (Efficient Funding of Chemotherapy) arrangement. While this application was accepted, the committee noted that on the basis of inadequate cost-benefit, the medicine would not be made available more generally for the first-line treatment of relapsed or refractory systemic anaplastic large cell lymphoma (sALCL).[7]
Design
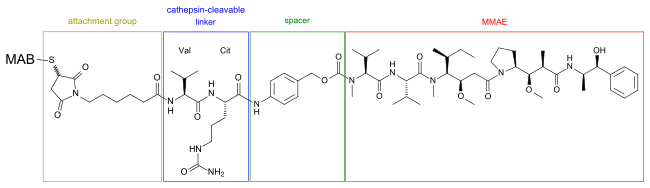
Brentuximab vedotin[8] consists of the chimeric monoclonal antibody brentuximab (cAC10, which targets the cell-membrane protein CD30) linked with maleimide attachment groups, cathepsin cleavable linkers (valine-citrulline), and para-aminobenzylcarbamate spacers to three to five units of the antimitotic agent monomethyl auristatin E (MMAE, reflected by the 'vedotin' in the drug's name).[9] The peptide-based linker bonds the antibody to the cytotoxic compound in a stable manner so the drug is not easily released from the antibody under physiologic conditions to help prevent toxicity to healthy cells and ensure dosage efficiency. The peptide antibody-drug bond facilitates rapid and efficient drug cleavage inside target tumor cell. The antibody cAC10 part of the drug binds to CD30 which often occurs on diseased cells but rarely on normal tissues. The antibody portion of the drug attaches to CD30 on the surface of malignant cells, delivering MMAE which is responsible for the anti-tumour activity.[10][11] Once bound, brentuximab vedotin is internalised by endocytosis and thus selectively taken up by targeted cells. The vesicle containing the drug is fused with lysosomes and lysosomal cysteine proteases, particularly cathepsin B, start to break down valine-citrulline linker and MMAE is no longer bound to the antibody and is released directly into the tumor environment. [12]
Clinical trials
In a 2010 clinical trial,[14] 34% of patients with refractory Hodgkin Lymphoma achieved complete remission and another 40% had partial remission.[15] Tumor reductions were achieved in 94% of patients. In ALCL, 87% of patients had tumors shrink at least 50% and 97% of patients had some tumor shrinkage.[16]
Reports in 2013 showed interim results[17] from a Phase II, open-label, single-arm study designed to evaluate the antitumor activity of brentuximab vedotin in relapsed or refractory CD30-positive NHL, including B-cell neoplasms. These results demonstrated that single-agent brentuximab vedotin induced a 42% objective response rate and manageable safety profile among advanced diffuse large B-cell lymphoma patients.[18][19]
A phase III trial funded by Millennium Pharmaceuticals compared ABVD (a combination of the chemotherapy drugs doxorubicin, bleomycin, vinblastine, and dacarbazine) versus A+AVD (a combination of brentuximab vedotin plus AVD, or doxorubicin, vinblastine, and dacarbazine) for treatment of classical Hodgkin lymphoma and found substituting brentuximab vedotin for bleomycin has both improved efficacy and lowered toxicity.[20] A previously completed phase I study demonstrated that a greater number of patients experienced pulmonary toxicity with brentuximab vedotin-ABVD than with ABVD alone. Pulmonary fibrosis is a classical adverse effect of bleomycin; however, the incidence of pulmonary fibrosis in the brentuximab vedotin-ABVD arm was higher than the expected historical rate with ABVD alone.[21] Overall, 24 out of 25 patients treated with brentuximab vedotin and AVD achieved complete remission.[22]
Brentuximab vedotin is also being investigated as a substitute for vincristine (another mitotic inhibitor which prevents tubulin polymerization) in patients with being treated with CHOP (a combination of cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone or prednisolone) for a non-Hodgkin lymphoma.
A phase III clinical trial is currently comparing the two combination therapies (CHOP and CHP-brentuximab vedotin) with estimated completion in December 2017.[23]
The ECHELON-1 phase 3 trial compared brentuximab vedotin with bleomycin both in combination with adriamycin, vinblastine, dacarbazine (AVD) chemotherapy as a firstline treatment for advanced classical Hodgkin lymphoma.[24]
Serious adverse events
Brentuximab vedotin was studied as monotherapy in 160 patients in two phase II trials. Across both trials, the most common adverse reactions (≥20%), regardless of causality, were chemotherapy-induced peripheral neuropathy (a progressive, enduring and often irreversible tingling numbness, intense pain, and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs), neutropenia (an immune system impairment), fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, fever, rash, thrombocytopenia, cough and vomiting.[25]
Black box warning
On January 13, 2012, the FDA announced that because brentuximab vedotin had been linked with two cases of progressive multifocal leukoencephalopathy, they were requiring the addition of a black box warning to the drug label regarding this potential risk.[26]
Interactions
Patients who are receiving strong CYP3A4 inhibitors concomitantly with brentuximab vedotin should be closely monitored for serious adverse events.[25]
Development and marketing collaboration
Brentuximab vedotin is marketed as Adcetris.[27] Seattle Genetics and Millennium Pharmaceuticals/Takeda Oncology are jointly developing brentuximab vedotin. Under the terms of the collaboration agreement, Seattle Genetics has U.S. and Canadian commercialization rights and the Takeda Group has rights to commercialize in the rest of the world. Seattle Genetics and the Takeda Group are funding joint development costs for brentuximab vedotin on a 50:50 basis, except in Japan where the Takeda Group will be solely responsible for development costs.
References
- Fierce Biotech: Seattle Genetics Submits BLA to FDA for brentuximab vedotin in relapsed or refractory hodgkin lymphoma and systemic ALCL
- U.S. FDA: Brentuximab Vedotin (marketed as Adcetris) Information
- Genetic Engineering & Biotechnology News: Seattle Genetics’ Antibody-Drug Conjugate Receives FDA Okay to Treat Lymphomas
- "FDA expands approval of Adcetris for first-line treatment of Stage III or IV classical Hodgkin lymphoma in combination with chemotherapy" (Press release). FDA. 20 March 2018. Retrieved 20 March 2018.
- EMA/European Medicines Agency: EPAR summary for the public for Adcetris/brentuximab vedotin
- "FDA Approves Brentuximab Vedotin for CTCL". OncLive. 2017. Retrieved 2017-11-10.
- PBAC Meetings March 2014 - Brentuximab Vedotin, 50 mg injection, 1 x 50 mg vial Adcetris® - March 2014 , March, 2014
- ADC Review / Journal of Antibody-drug Conjugates: Brentuximab Vedotin, February 18, 2014
- ADC Review / Journal of Antibody-drug Conjugates: Monomethyl auristatin E (MMAE), May 23, 2013
- Seattle Genetics: Clinical Trials with brentuximab vedotin (SGN-35) Archived 2011-07-16 at the Wayback Machine
- Francisco, J. A.; Cerveny, C. G.; Meyer, D. L.; Mixan, B. J.; Klussman, K.; Chace, D. F.; Rejniak, S. X.; Gordon, K. A.; Deblanc, R.; Toki, B. E.; Law, C. L.; Doronina, S. O.; Siegall, C. B.; Senter, P. D.; Wahl, A. F. (2003). "CAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity". Blood. 102 (4): 1458–1465. doi:10.1182/blood-2003-01-0039. PMID 12714494.
- Christos Vaklavas and Andres Forero-Torres; Safety and efficacy of brentuximab vedotin in patients with Hodgkin lymphoma or systemic anaplastic large cell lymphoma Therapeutic Advances in Hematology (August 2012) vol. 3 no. 4: 209-225 doi: 10.1177/2040620712443076
- A. Klement (13 May 2013). "Sprunginnovation beim Hodgkin-Lymphom: Adcetris". Österreichische Apothekerzeitung (in German) (10/2013): 68.
- Clinical trial number NCT00848926 for "A Pivotal Open-Label Trial of Brentuximab Vedotin for Hodgkin Lymphoma" at ClinicalTrials.gov
- Seattle Genetics and Millennium Report Positive Data from Pivotal Trial of Brentuximab Vedotin (SGN-35) in Relapsed or Refractory Hodgkin Lymphoma at 2010 Annual Meeting of the American Society of Hematology (ASH) (Corporate Press Release)
- Minyanville Business News: Is Seattle Genetics the Next Big Thing?, December 2, 2010
- Jeff P. Sharman (21 October 2013). "A Phase 2 Study Of Brentuximab Vedotin In Patients With Relapsed Or Refractory CD30-Positive Non-Hodgkin Lymphomas: Interim Results In Patients With DLBCL and Other B-Cell Lymphomas". Blood. 122 (21): 848. doi:10.1182/blood.V122.21.848.848.
- Clinical trial number NCT01421667 for "A Study of Brentuximab Vedotin in Relapsed or Refractory Non-Hodgkin Lymphoma" at ClinicalTrials.gov
- "Brentuximab Vedotin Shows 42% Objective Response Rate in Patients with Relapsed or Refractory Diffuse Large B-cell Lymphoma, Study Shows". ADC Review / Journal of Antibody-drug Conjugates. 10 December 2013. Archived from the original on 17 December 2013.
- Connors, Joseph M.; Jurczak, Wojciech; Straus, David J.; Ansell, Stephen M.; Kim, Won S.; Gallamini, Andrea; Younes, Anas; Alekseev, Sergey; Illés, Árpád (January 25, 2018). "Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma". The New England Journal of Medicine. 378 (4): 331–344. doi:10.1056/NEJMoa1708984. ISSN 1533-4406. PMC 5819601. PMID 29224502.
- Research, Center for Drug Evaluation and. "Drug Safety and Availability - FDA Drug Safety Communication: New Boxed Warning and Contraindication for Adcetris (brentuximab vedotin)". www.fda.gov. Retrieved 2017-08-04.
- Anas Younes, Joseph M. Connors, Steven I. Park, Michelle Fanale, Megan M. O'Meara, Naomi N. Hunder et al; Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study The Lancet Oncology, (December 2013) Volume 14, Issue 13, 1348 - 1356 doi:10.1016/S1470-2045(13)70501-1
- A Randomized, Double-blind, Placebo-controlled, Phase 3 Study of Brentuximab Vedotin and CHP (A+CHP) Versus CHOP in the Frontline Treatment of Patients With CD30-positive Mature T-cell Lymphomas trial identifier: NCT01777152
- Seattle Genetics' Adcetris succeeds in study but shares slide. June 2017
- Highlights of Prescribing Information (US)/Adcetris (brentuximab vedotin) for Injection Archived May 25, 2013, at the Wayback Machine (2012)
- Adcetris (brentuximab vedotin): Drug Safety Communication - Progressive Multifocal Leukoencephalopathy and Pulmonary Toxicity
- Onco'Zine - The International Cancer Network: European Medicines Agency Accepts Brentuximab Marketing Authorization Application Archived 2013-10-29 at the Wayback Machine, June 27, 2011