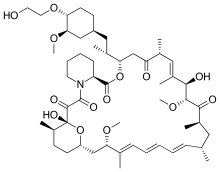
Everolimus
Everolimus is the 40-O-(2-hydroxyethyl) derivative of sirolimus and works similarly to sirolimus as an inhibitor of mammalian target of rapamycin (mTOR).
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Everolimus /ˌɛvəˈroʊləməs/ |
| Trade names | Afinitor, Zortress |
| Other names | 42-O-(2-hydroxyethyl)rapamycin, RAD001 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609032 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | ~30 hours[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.896 |
| Chemical and physical data | |
| Formula | C53H83NO14 |
| Molar mass | 958.224 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It is currently used as an immunosuppressant to prevent rejection of organ transplants and in the treatment of renal cell cancer and other tumours. Much research has also been conducted on everolimus and other mTOR inhibitors as targeted therapy for use in a number of cancers.
It is marketed by Novartis under the trade names Zortress (USA) and Certican (Europe and other countries) in transplantation medicine, and as Afinitor (general tumours) and Votubia (tumours as a result of TSC) in oncology. Everolimus is also available from Biocon, with the brand name Evertor.
Medical uses
Everolimus is approved for various conditions:
- Advanced kidney cancer (US FDA approved in March 2009)[3]
- Prevention of organ rejection after renal transplant(US FDA April 2010)[4]
- Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis (TS) in patients who are not suitable for surgical intervention (US FDA October 2010)[5]
- Progressive or metastatic pancreatic neuroendocrine tumors not surgically removable (May 2011)[6]
- Breast cancer in post-menopausal women with advanced hormone-receptor positive, HER2-negative type cancer, in conjunction with exemestane (US FDA July 2012)[7]
- Prevention of organ rejection after liver transplant(Feb 2013)
- Progressive, well-differentiated non-functional, neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin with unresectable, locally advanced or metastatic disease (US FDA February 2016).[8]
- Tuberous sclerosis complex-associated partial-onset seizures for adult and pediatric patients aged 2 years and older. (US FDA April 2018).[9]
UK National Health Service
NHS England has been criticised for delays in deciding on a policy for the prescription of everolimus in the treatment of Tuberous Sclerosis. 20 doctors addressed a letter to the board in support of the charity Tuberous Scelerosis Association saying " around 32 patients with critical need, whose doctors believe everolimus treatment is their best or only option, have no hope of access to funding. Most have been waiting many months. Approximately half of these patients are at imminent risk of a catastrophic event (renal bleed or kidney failure) with a high risk of preventable death."[10] In May 2015 it was reported that Luke Henry and Stephanie Rudwick, the parents of a child suffering from Tuberous Sclerosis were trying to sell their home in Brighton to raise £30,000 to pay for treatment for their daughter Bethany who has tumours on her brain, kidneys and liver and suffers from up to 50 epileptic fits a day.[11]
Clinical trials
As of October 2010, Phase III trials are under way in gastric cancer, hepatocellular carcinoma, and lymphoma.[12] The experimental use of everolimus in refractory chronic graft-versus-host disease was reported in 2012.[13]
Interim phase III trial results in 2011 showed that adding Afinitor (everolimus) to exemestane therapy against advanced breast cancer can significantly improve progression-free survival compared with exemestane therapy alone.[14]
A study published in 2012 shows that everolimus sensitivity varies between patients depending on their tumor genomes.[15] A group of patients with advanced metastasic bladder carcinoma (NCT00805129) [16] treated with everolimus revealed a single patient who had a complete response to everolimus treatment for 26 months. The researchers sequenced the genome of this patient and compared it to different reference genomes and to other patients' genomes. They found that mutations in TSC1 led to a lengthened duration of response to everolimus and to an increase in the time to cancer recurrence. The mutated TSC1 apparently had made these tumors vulnerable to treatment with everolimus.
Mechanism
Compared with the parent compound rapamycin, everolimus is more selective for the mTORC1 protein complex, with little impact on the mTORC2 complex.[17] This can lead to a hyper-activation of the kinase AKT via inhibition on the mTORC1 negative feedback loop, while not inhibiting the mTORC2 positive feedback to AKT. This AKT elevation can lead to longer survival in some cell types. Thus, everolimus has important effects on cell growth, cell proliferation and cell survival.
Additionally, mTORC2 is believed to play an important role in glucose metabolism and the immune system, suggesting that selective inhibition of mTORC1 by drugs such as everolimus could achieve many of the benefits of rapamycin without the associated glucose intolerance and immunosuppression.[17]
TSC1 and TSC2, the genes involved in tuberous sclerosis, act as tumor suppressor genes by regulating mTORC1 activity. Thus, either the loss or inactivation of one of these genes lead to the activation of mTORC1.[18]
Everolimus binds to its protein receptor FKBP12, which directly interacts with mTORC1, inhibiting its downstream signaling. As a consequence, mRNAs that code for proteins implicated in the cell cycle and in the glycolysis process are impaired or altered, and tumor growth is inhibited.[18]
Adverse reactions
A trial using 10 mg/day in patients with NETs of GI or lung origin reported "Everolimus was discontinued for adverse reactions in 29% of patients and dose reduction or delay was required in 70% of everolimus-treated patients. Serious adverse reactions occurred in 42% of everolimus-treated patients and included 3 fatal events (cardiac failure, respiratory failure, and septic shock). The most common adverse reactions (incidence greater than or equal to 30%) were stomatitis, infections, diarrhea, peripheral edema, fatigue and rash. The most common blood abnormalities found (incidence greater than or equal to 50%) were anemia, hypercholesterolemia, lymphopenia, elevated aspartate transaminase (AST) and fasting hyperglycemia.".[8]
Role in heart transplantation
Everolimus may have a role in heart transplantation, as it has been shown to reduce chronic allograft vasculopathy in such transplants. It also may have a similar role to sirolimus in kidney and other transplants.[19]
Role in liver transplantation
Although, sirolimus had generated fears over use of m-TOR inhibitors in liver transplantation recipients, due to possible early hepatic artery thrombosis and graft loss, use of everolimus in the setting of liver transplantation is promising. Jeng et al.,[20] in their study of 43 patients, concluded the safety of everolimus in the early phase after living donor liver transplantation. In their study, no hepatic artery thrombosis or wound infection was noted. Also, a possible role of everolimus in reducing the recurrence of hepatocellular carcinoma after liver transplantation was correlated. A target trough level of 3 ng/mL at 3 months was shown to be beneficial in recipients with pre-transplant renal dysfunction. In their study, 6 of 9 renal failure patients showed significant recovery of renal function, whereas 3 showed further deterioration, one of whom required hemodialysis.[21] Recently published report by Thorat et al showed a positive impact on hepatocellular carcinoma (HCC) when everolimus was used as primary immunosuppression starting as early as first week after living donor liver transplantation (LDLT) surgery.[22] In their retrospective and prospective analysis at China Medical University Hospital in Taiwan, the study cohort (n=66) was divided in two groups depending upon the postoperative immunosuppression. Group A: HCC patients that received Everolimus + Tacrolimus based immunosuppressive regimen (n=37). Group B: HCC patients that received standard Tacrolimus based immunosuppressive regimen without everolimus (n=29). The target trough level for EVR was 3 to 5 ng/ml while for TAC it was 8–10 ng/ml. The 1-year, 3-year and 4-year overall survival achieved for Group A patients (Everolimus group) was 94.95%, 86.48% and 86.48%, respectively while for Group B patients it was 82.75%, 68.96%, and 62.06%, respectively (p=0.0217). The first 12-month report of ongoing Everolimus multicenter prospective trial in LDLT (H2307 trial), Jeng LB et al have shown a 0% recurrence of HCC in everolimus group at 12 months.[23] Jeng LB concluded that an early introduction of everolimus + reduced tacrolimus was non-inferior to standard tacrolimus in terms of efficacy and renal function at 12 months, with HCC recurrence only in tacrolimus control patients.
Use in vascular stents
Everolimus is used in drug-eluting coronary stents as an immunosuppressant to prevent restenosis. Abbott Vascular produce an everolimus-eluting stent (EES) called Xience Alpine. It utilizes the Multi-Link Vision cobalt chromium stent platform and Novartis' everolimus. The product is widely available globally including USA, Europe, and APAC countries. Boston Scientific also market EESes, recent offerings being Promus Elite and Synergy.
Use in aging
Inhibition of mTOR, the molecular target of everolimus, extends the lifespan of model organisms including mice,[24] and mTOR inhibition has been suggested as an anti-aging therapy. Everolimus was used in a recent clinical trial by Novartis, and short-term treatment was shown to enhance the response to the influenza vaccine in the elderly, possible by reversing immunosenescence.[25] Everolimus treatment of mice results in reduced metabolic side effects compared to sirolimus.[17]
References
- Use During Pregnancy and Breastfeeding
- Formica RN, Lorber KM, Friedman AL, Bia MJ, Lakkis F, Smith JD, Lorber MI (March 2004). "The evolving experience using everolimus in clinical transplantation". Transplantation Proceedings. 36 (2 Suppl): 495S–499S. doi:10.1016/j.transproceed.2004.01.015. PMID 15041395.
- "Afinitor approved in US as first treatment for patients with advanced kidney cancer after failure of either sunitinib or sorafenib" (Press release). Novartis. 30 March 2009. Retrieved 6 April 2009.
- "Novartis receives US FDA approval for Zortress (everolimus) to prevent organ rejection in adult kidney transplant recipients" (Press release). Novartis. 22 April 2010. Archived from the original on 25 April 2010. Retrieved 26 April 2010.
- "Novartis' Afinitor Cleared by FDA for Treating SEGA Tumors in Tuberous Sclerosis". 1 November 2010.
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm254350.htm
- "US FDA approves Novartis drug Afinitor for breast cancer". Reuters. 20 July 2012.
- Everolimus (Afinitor). Feb 2016
- Everolimus (Afinitor). April 2018
- Lintern, Shaun (14 April 2015). "Policy delays risk 'preventable deaths', doctors warn NHS England". Health Service Journal. Retrieved 20 April 2015.
- "Couple forced to sell home after NHS refuse to fund daughter's treatment for rare illness". Daily Express. 11 May 2015. Retrieved 12 May 2015.
- http://www.genengnews.com/gen-news-highlights/novartis-afinitor-cleared-by-fda-for-treating-sega-tumors-in-tuberous-sclerosis/81244159/
- Lutz M, Kapp M, Grigoleit GU, Stuhler G, Einsele H, Mielke S (April 2012). "Salvage therapy with everolimus improves quality of life in patients with refractory chronic graft-versus-host disease" (PDF). Bone Marrow Transplant. 47 (S1): S410–S411.
- "Positive Trial Data Leads Novartis to Plan Breast Cancer Filing for Afinitor by Year End". 2011.
- Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, Pirun M, Sander C, Socci ND, Ostrovnaya I, Viale A, Heguy A, Peng L, Chan TA, Bochner B, Bajorin DF, Berger MF, Taylor BS, Solit DB (October 2012). "Genome sequencing identifies a basis for everolimus sensitivity". Science. 338 (6104): 221. doi:10.1126/science.1226344. PMC 3633467. PMID 22923433.
- Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW (February 2016). "Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system". Aging Cell. 15 (1): 28–38. doi:10.1111/acel.12405. PMC 4717280. PMID 26463117.
- "Archived copy". Archived from the original on 8 March 2014. Retrieved 26 February 2014.CS1 maint: archived copy as title (link)
- Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM, Abeywickrama KH, Bernhardt P (August 2003). "Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients". The New England Journal of Medicine. 349 (9): 847–58. doi:10.1056/NEJMoa022171. PMID 12944570.
- Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, Hsu SC, Hsu CH (April 2014). "Experience of using everolimus in the early stage of living donor liver transplantation". Transplantation Proceedings. 46 (3): 744–8. doi:10.1016/j.transproceed.2013.11.068. PMID 24767339.
- Jeng L, Thorat A, Yang H, Yeh C-C, Chen T-H, Hsu S-C. Impact of Everolimus On the Hepatocellular Carcinoma Recurrence After Living Donor Liver Transplantation When Used in Early Stage: A Single Center Prospective Study [abstract]. Am J Transplant. 2015; 15 (suppl 3). http://www.atcmeetingabstracts.com/abstract/impact-of-everolimus-on-the-hepatocellular-carcinoma-recurrence-after-living-donor-liver-transplantation-when-used-in-early-stage-a-single-center-prospective-study/. Accessed 1 September 2015.
- Thorat A, Jeng LB, Yang HR, Yeh CC, Hsu SC, Chen TH, Poon KS (November 2017). "Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living donor liver transplantation for patients within the UCSF criteria: re-inventing the role of mammalian target of rapamycin inhibitors". Annals of Hepato-Biliary-Pancreatic Surgery. 21 (4): 205–211. doi:10.14701/ahbps.2017.21.4.205. PMC 5736740. PMID 29264583.
- Jeng LB, Lee SG, Soin AS, Lee WC, Suh KS, Joo DJ, Uemoto S, Joh J, Yoshizumi T, Yang HR, Song GW, Lopez P, Kochuparampil J, Sips C, Kaneko S, Levy G (December 2017). "Efficacy and safety of everolimus with reduced tacrolimus in living-donor liver transplant recipients: 12-month results of a randomized multicenter study". American Journal of Transplantation. 18 (6): 1435–1446. doi:10.1111/ajt.14623. PMID 29237235.
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (July 2009). "Rapamycin fed late in life extends lifespan in genetically heterogeneous mice". Nature. 460 (7253): 392–5. doi:10.1038/nature08221. PMC 2786175. PMID 19587680.
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB (December 2014). "mTOR inhibition improves immune function in the elderly". Science Translational Medicine. 6 (268): 268ra179. doi:10.1126/scitranslmed.3009892. PMID 25540326.
Further reading
- Sedrani R, Cottens S, Kallen J, Schuler W (August 1998). "Chemical modification of rapamycin: the discovery of SDZ RAD". Transplantation Proceedings. 30 (5): 2192–4. doi:10.1016/S0041-1345(98)00587-9. PMID 9723437.
External links
- "Everolimus". Drug Information Portal. U.S. National Library of Medicine.