Selumetinib
Selumetinib (AZD6244) is a drug that was discovered by Array BioPharma and was licensed to AstraZeneca. It is being investigated for the treatment of various types of cancer, such as non-small cell lung cancer (NSCLC) and thyroid cancer.[1][2]
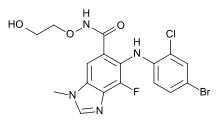 | |
| Clinical data | |
|---|---|
| Other names | AZD6244, ARRY-142886 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.311 |
| Chemical and physical data | |
| Formula | C17H15BrClFN4O3 |
| Molar mass | 457.68 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mechanism of action
The gene BRAF is part of the MAPK/ERK pathway, a chain of proteins in cells that communicates input from growth factors. Activating mutations in the BRAF gene, primarily V600E (meaning that the amino acid valine in position 600 is replaced by glutamic acid), are associated with lower survival rates in patients with papillary thyroid cancer. Another type of mutation that leads to undue activation of this pathway occurs in the gene KRAS and is found in NSCLC. A possibility of reducing the activity of the MAPK/ERK pathway is to block the enzyme MAPK kinase (MEK), immediately downstream of BRAF, with the drug selumetinib. More specifically, selumetinib blocks the subtypes MEK1 and MEK2 of this enzyme.[3]
Possible uses
Treatment of neurofibromas in those with neurofibromatosis.
In addition to thyroid cancer, BRAF-activating mutations are prevalent in melanoma (up to 59%), colorectal cancer (5–22%), serous ovarian cancer (up to 30%), and several other tumor types.[4]
Selumetinib has also been shown to inhibit growth of GNAQ mutated uveal melanoma cell lines.[5] Furthermore, preliminary results suggest that selumetinib treatment of uveal melanoma patients can result in tumor shrinkage as the consequence of sustained inhibition of ERK phosphorylation.[6]
KRAS mutations appear in 20 to 30% of NSCLC cases and about 40% of colorectal cancer.[3]
A Phase II clinical trial about selumetinib in NSCLC has been completed in September 2011;[7] one about cancers with BRAF mutations is ongoing as of June 2012.[8]
In July 2015 selumetinib failed a Phase III trial testing whether the drug significantly prolonged the survival of patients in a study on melanoma originating in the eye. In the 152-patient trial, a combination of selumetinib and dacarbazine failed to improve progression-free survival compared with just the old drug alone.[9][10]
As of March 2016 there are other phase 3 trials registered for thyroid cancer,[11] and KRAS Positive NSCLC.[12] The combination of selumetinib to chemotherapy improved median progression-free survival }}in a trial of 510 patients with advanced KRAS-mutant non–small cell lung cancer (NSCLC)just for one month, which was statistically not significant.[13]
In November 2018, investigators working with nasal polyp tissue in vitro demonstrated a synergistic effect of down regulating expression of p-MEK1 and p-ERK1 when it was administered with erythromycin.
References
- "Array BioPharma strikes rights deal with Japanese firm worth up to $76M-plus – BizWest". BizWest. 2016-03-31. Retrieved 2018-06-12.
- Casaluce F, Sgambato A, Maione P, Sacco PC, Santabarbara G, Gridelli C (August 2017). "Selumetinib for the treatment of non-small cell lung cancer". Expert Opinion on Investigational Drugs. 26 (8): 973–984. doi:10.1080/13543784.2017.1351543. PMID 28675058.
- Troiani T, Vecchione L, Martinelli E, Capasso A, Costantino S, Ciuffreda LP, et al. (May 2012). "Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells". British Journal of Cancer. 106 (10): 1648–59. doi:10.1038/bjc.2012.129. PMC 3349172. PMID 22569000.
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. (June 2002). "Mutations of the BRAF gene in human cancer" (PDF). Nature. 417 (6892): 949–54. doi:10.1038/nature00766. PMID 12068308.
- Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK (July 2012). "Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance". Clinical Cancer Research. 18 (13): 3552–61. doi:10.1158/1078-0432.CCR-11-3086. PMC 3433236. PMID 22550165.
- "Pharmacodynamic activity of selumetinib to predict radiographic response in advanced uveal melanoma". 2012.
- Clinical trial number NCT00890825 for "Comparison of AZD6244 in Combination With Docetaxel Versus Docetaxel Alone in KRAS Mutation Positive Non Small Cell Lung Cancer (NSCLC) Patients" at ClinicalTrials.gov
- Clinical trial number NCT00888134 for "AZD6244 in Cancers With BRAF Mutations" at ClinicalTrials.gov
- "AstraZeneca - AstraZeneca provides update on selumetinib in uveal melanoma". astrazeneca.com.
- "AstraZeneca's once-lauded drug flunks a Phase III eye cancer trial". FierceBiotech.
- {{ClinicalTrialsGov|NCT01843062|Study Comparing Complete Remission After Treatment With Selumetinib/Placebo in Patient With Differentiated Thyroid Cancer (ASTRA)
- Clinical trial number NCT01933932 for "Assess Efficacy & Safety of Selumetinib in Combination With Docetaxel in Patients Receiving 2nd Line Treatment for v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Positive NSCLC (SELECT-1)" at ClinicalTrials.gov
- Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. (May 2017). "Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial". JAMA. 317 (18): 1844–1853. doi:10.1001/jama.2017.3438. PMC 5815037. PMID 28492898.
Further reading
- Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. (February 2013). "Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer". The New England Journal of Medicine. 368 (7): 623–32. doi:10.1056/NEJMoa1209288. PMC 3615415. PMID 23406027.