Acalabrutinib
Acalabrutinib (trade name Calquence) is a medication used to treat a type of non-Hodgkin lymphoma known as mantle cell lymphoma.[1] Specifically it is for people who had previously been treated with another therapy.[2] It is unclear if it results in improved outcomes as of 2019.[1]
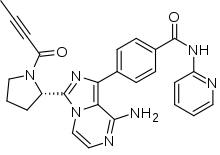 | |
| Clinical data | |
|---|---|
| Trade names | Calquence |
| Other names | ACP-196 |
| AHFS/Drugs.com | Monograph |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.247.121 |
| Chemical and physical data | |
| Formula | C26H23N7O2 |
| Molar mass | 465.517 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Common side effects include headaches, feeling tired, low red blood cells, low platelets, and low white blood cells.[1] It is a second generation Bruton's tyrosine kinase inhibitor.[3][4]
Acalabrutinib was approved for medical use in the United States in 2017.[1] The whole sale cost as of 2018 in the United States is 14,064 USD per month.[5]
Medical uses
It is used to treat a type of non-Hodgkin lymphoma known as mantle cell lymphoma.[1] It is unclear if it results in improved outcomes as of 2019.[1]
Side effects
The most common adverse events were headache, diarrhea and weight gain.[4] Despite the appearance of a greater occurrence of transient headaches, data suggests a preferred advantage of acalabrutinib over ibrutinib due to expected reduced adverse events of skin rash, severe diarrhea, and bleeding risk.[4]
Society and culture
Regulatory
As of February 2016, acalabrutinib had received orphan designation in the United States for CLL only,[7] and was similarly designated as an orphan medicinal product by the European Medicines Agency (EMA) Committee for Orphan Medicinal Products (COMP) for treatment of three indications - chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), and lymphoplasmacytic lymphoma (Waldenström's macroglobulinaemia, WM).[8] If the drug is ultimately approved, this designation will result in a 10-year period of market exclusivity for the stated indications within Europe.[9]
Economics
It was developed by Acerta Pharma.[10] After the promising results for the treatment of CLL in initial clinical trials,[3] Astra Zeneca purchased a 55% stake in Acerta Pharma for $4 billion in December 2015, with an option to acquire the remaining 45% stake for an additional $3 billion, conditional on the first approval in both the US and Europe and the establishment of commercial opportunity.[11]
Research
Relative to ibrutinib, acalabrutinib demonstrated higher selectivity and inhibition of the targeted activity of BTK, while having a much greater IC50 or otherwise virtually no inhibition on the kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1.[4] In addition, in platelets treated with ibrutinib, thrombus formation was clearly inhibited while no impact to thrombus formation was identified relative to controls for those treated with acalabrutinib.[4] These findings strongly suggest an improved safety profile of acalabrutinib with minimized adverse effects relative to ibrutinib.[4] In pre-clinical studies, it was shown to be more potent and selective than ibrutinib, the first-in-class BTK inhibitor.[3][4][12]
The interim results of the still on-going first human phase 1/2 clinical trial (NCT02029443) with 61 patients for the treatment of relapsed chronic lymphocytic leukemia (CLL) are encouraging, with a 95% overall response rate demonstrating potential to become a best-in-class treatment for CLL.[3] Notably, a 100% response rate was achieved for those people which were positive for the 17p13.1 gene deletion - a subgroup that typically results in a poor response to therapy and expected outcomes.[4]
References
- "Acalabrutinib Monograph for Professionals". Drugs.com. Retrieved 16 March 2019.
- "Press Announcements - FDA approves new treatment for adults with mantle cell lymphoma".
- Byrd; et al. (2015). "Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia". New England Journal of Medicine. 374 (4): 323–332. doi:10.1056/NEJMoa1509981. PMC 4862586. PMID 26641137.
- Wu, Jingjing; Zhang, Mingzhi; Liu, Delong (2016-01-01). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9: 21. doi:10.1186/s13045-016-0250-9. ISSN 1756-8722. PMC 4784459. PMID 26957112.
- "In Brief: Acalabrutinib (Calquence) for Mantle Cell Lymphoma (online only) | The Medical Letter, Inc". secure.medicalletter.org. Retrieved 16 March 2019.
- "WHO Drug Information - recommended INN" (PDF). WHO Drug Information. World Health Oorganisation. Retrieved 24 December 2015.
- "Public summary of opinion on orphan designation" (PDF). European Medicines Agency. 2016-04-27. Retrieved 2016-11-20.
- "azn201602256k.htm". www.sec.gov. Retrieved 2016-11-21.
- House, SA Editor Douglas W. (2016-02-25). "AstraZeneca and Acerta Pharma's acalabrutinib tagged an Orphan Drug in Europe for three indications". Seeking Alpha. Retrieved 2016-11-21.
- "AstraZeneca to buy Acerta for blood cancer drug". www.rsc.org. Chemistry World - Royal Society of Chemistry. Retrieved 24 December 2015.
- Walker, Ian; Roland, Denise (2015-12-17). "AstraZeneca to Buy Stake in Acerta Pharma". Wall Street Journal. ISSN 0099-9660. Retrieved 2016-11-19.
- Wu, Jingjing; Zhang, Mingzhi; Liu, Delong (2016-03-09). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9 (1). doi:10.1186/s13045-016-0250-9. ISSN 1756-8722. PMC 4784459. PMID 26957112.