Azaprocin
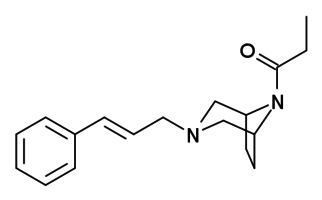
Azaprocin is a drug which is an opioid analgesic with approximately ten times the potency of morphine, and a fast onset and short duration of action.[1][2][3] It was discovered in 1963, but has never been marketed.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H24N2O |
| Molar mass | 284.396 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 170 to 175 °C (338 to 347 °F) |
SMILES
| |
InChI
| |
| | |
The derivative substituted on the phenyl ring with a p-nitro group is more potent than the parent compound, around 25x the potency of morphine.[4] The ring-opened 2,6-dimethylpiperazine analogues are also active,[5] and a large family of opioid analgesic compounds derived from this parent structure have been developed over the last 40 years.[6][7][8][9][10][11][12][13][14][15] One analogue, AP-237, has been used in China to treat the pain caused by cancer.
References
- Cignarella, G; Occelli, E; Cristiani, G; Paduano, L; Testa, E (1963). "Bicyclic Homologs of Piperazine. Vi. Synthesis and Analgesic Activity of 3-Substituted 8-Propionyl-3,8-Diazabicyclo(3.2.1)Octanes". Journal of Medicinal Chemistry. 6 (6): 764–6. doi:10.1021/jm00342a030. PMID 14184943.
- Cignarella, G; Occelli, E; Testa, E (1965). "Bicyclic Homologs of Piperazine. Vii. Synthesis and Analgesic Activity of 3-Aralkenyl-8-Propionyl-3,8-Diazabicyclo(3.2.1)Octanes". Journal of Medicinal Chemistry. 8 (3): 326–31. doi:10.1021/jm00327a010. PMID 14323140.
- Rosselli Del Turco, B; Maffii, G (1968). "Effect of analgesic drugs on the conditioned behavior of rats". Bollettino Chimico Farmaceutico. 107 (2): 120–6. PMID 5730115.
- Cignarella, G; Barlocco, D; Tranquillini, M. E.; Volterra, A; Brunello, N; Racagni, G (1988). "Interaction of 3,8-diazabicyclo (3.2.1) octanes with mu and delta opioid receptors". Pharmacological Research Communications. 20 (5): 383–94. doi:10.1016/s0031-6989(88)80014-6. PMID 2843931.
- Cignarella, G; Testa, E (1968). "2,6-Dialkylpiperazines. IV. 1-Propionyl-4-substituted cis-2,6-dimethylpiperazines structurally related to the analgetic 8-acyl-3,8-diazabicyclo3.2.1octanes". Journal of Medicinal Chemistry. 11 (3): 592–4. doi:10.1021/jm00309a039. PMID 5656502.
- Cignarella, G; Barlocco, D; Tranquillini, M. E.; Volterra, A; Brunello, N; Racagni, G (1988). "Interaction of 3,8-diazabicyclo (3.2.1) octanes with mu and delta opioid receptors". Pharmacological Research Communications. 20 (5): 383–94. doi:10.1016/s0031-6989(88)80014-6. PMID 2843931.
- Barlocco, D; Cignarella, G; Greco, G; Novellino, E (1993). "Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo3.2.1octane derivatives binding to the mu-opioid receptor". Journal of Computer-aided Molecular Design. 7 (5): 557–71. doi:10.1007/bf00124362. PMID 8294946.
- Fadda, P; Barlocco, D; Tronci, S; Cignarella, G; Fratta, W (1997). "Antinociceptive action of DBO 17 and DBO 11 in mice: Two 3,8 diazabicyclo (3.2.1.) octane derivates with selective mu opioid receptor affinity". Naunyn-Schmiedeberg's Archives of Pharmacology. 356 (5): 596–602. doi:10.1007/pl00005095. PMID 9402039.
- Barlocco, D; Cignarella, G; Vianello, P; Villa, S; Pinna, G. A.; Fadda, P; Fratta, W (1998). "Synthesis and mu-opioid receptor affinity of a new series of nitro substituted 3,8-diazabicyclo3.2.1octane derivatives". Farmaco. 53 (8–9): 557–62. doi:10.1016/s0014-827x(98)00065-2. PMID 10081818.
- Cignarella, G; Barlocco, D; Vianello, P; Villa, S; Pinna, G. A.; Fadda, P; Fratta, W; Toma, L; Gessi, S (1998). "Benzocondensed derivatives as rigid analogues of the mu-opioid agonist 3(8)-cinnamyl-8(3)-propionyl-3,8-diazabicyclo3.2.1octanes: Synthesis, modeling, and affinity". Farmaco. 53 (10–11): 667–74. doi:10.1016/s0014-827x(98)00084-6. PMID 10205853.
- Vianello, P; Albinati, A; Pinna, G. A.; Lavecchia, A; Marinelli, L; Borea, P. A.; Gessi, S; Fadda, P; Tronci, S; Cignarella, G (2000). "Synthesis, molecular modeling, and opioid receptor affinity of 9, 10-diazatricyclo4.2.1.1(2,5)decanes and 2,7-diazatricyclo4.4.0. 0(3,8)decanes structurally related to 3,8-diazabicyclo3.2. 1octanes". Journal of Medicinal Chemistry. 43 (11): 2115–23. doi:10.1021/jm991140q. PMID 10841790.
- Pinna, G. A.; Murineddu, G; Curzu, M. M.; Villa, S; Vianello, P; Borea, P. A.; Gessi, S; Toma, L; Colombo, D; Cignarella, G (2000). "Synthesis, modelling, and mu-opioid receptor affinity of N-3(9)-arylpropenyl-N-9(3)-propionyl-3,9-diazabicycl". Farmaco. 55 (8): 553–62. doi:10.1016/s0014-827x(00)00036-7. PMID 11132733.
- Pinna, G. A.; Cignarella, G; Loriga, G; Murineddu, G; Mussinu, J. M.; Ruiu, S; Fadda, P; Fratta, W (2002). "N-3(9)-arylpropenyl-N-9(3)-propionyl-3,9-diazabicyclo3.3.1nonanes as mu-opioid receptor agonists. Effects on mu-affinity of arylalkenyl chain modifications". Bioorganic & Medicinal Chemistry. 10 (6): 1929–37. doi:10.1016/s0968-0896(01)00436-9. PMID 11937351.
- Pinna, G. A.; Cignarella, G; Ruiu, S; Loriga, G; Murineddu, G; Villa, S; Grella, G. E.; Cossu, G; Fratta, W (2003). "Synthesis of novel diazatricyclodecanes (DTDs). Effects of structural variation at the C3' allyl end and at the phenyl ring of the cinnamyl chain on mu-receptor affinity and opioid antinociception". Bioorganic & Medicinal Chemistry. 11 (18): 4015–26. doi:10.1016/s0968-0896(03)00373-0. PMID 12927864.
- Loriga, G; Manca, I; Murineddu, G; Chelucci, G; Villa, S; Gessi, S; Toma, L; Cignarella, G; Pinna, G. A. (2006). "Synthesis of 3,6-diazabicyclo3.1.1heptanes as novel ligands for the opioid receptors". Bioorganic & Medicinal Chemistry. 14 (3): 676–91. doi:10.1016/j.bmc.2005.09.045. PMID 16243530.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.