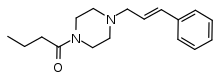
Bucinnazine
Bucinnazine (AP-237, 1-butyryl-4-cinnamylpiperazine) is an opioid analgesic drug that was widely used in China to treat pain in cancer patients as of 1986.[1] It is one of the most potent compounds among a series of analgesic acyl piperazine compounds first synthesized and reported in Japan in the 1970s.[2][3][4] Bucinnazine has analgesic potency comparable to that of morphine but with a relatively higher therapeutic index.
 | |
| Clinical data | |
|---|---|
| Other names | AP-237 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H24N2O |
| Molar mass | 272.392 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The drug was initially claimed to be a non-narcotic analgesic. However, subsequent studies have shown bucinnazine and similar acyl piperazines to be potent and selective agonists of μ-opioid receptor (MOR) with relatively low affinity for the δ-opioid receptor and the κ-opioid receptor.[5] In accordance with these studies, results from the intravenous self-administration experiments in rats showed that bucinnazine has a marked reinforcing effect with tolerance and dependence quickly developing.[1] In addition, the morphine antagonist naloxone reverses the effect of bucinnazine and precipitates withdrawal symptoms in bucinnazine treated rats further indicating a mechanism of analgesia mediated via selective agonist activity at μ-opioid receptors.
Derivatives
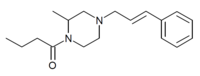
The 2-methyl and 2,6-dimethyl derivatives of AP-237 are also known to be active as opioid analgesics,[6][7] and 2-methyl-AP-237 has been sold as a designer drug, first identified by a police forensic laboratory in Slovenia in March 2019.[8]
References
- Qing T, Zhi-Ji C, Wei-Ping W (1986). "Experimental Study on the Dependence-Producing Properties of Qiang Tong Ding (AP-237)". Chin. J. Clin. Pharmacol. (2).
- Nishimura, N.; Kiuchi, M.; Kanetake, Y.; Takahashi, T. (1970). "Clinical exaluation of a new analgesic agent Ap-237". Masui. The Japanese Journal of Anesthesiology. 19 (6): 653–6. PMID 4916908.
- Carrano, R. A.; Kimura, K. K.; McCurdy, D. H. (1975). "Analgesic and tolerance studies with AP-237, a new analgesic". Archives Internationales de Pharmacodynamie et de Therapie. 213 (1): 41–57. PMID 1156018.
- Carrano, R. A.; Kimura, K. K.; Landes, R. C.; McCurdy, D. H. (1975). "General pharmacology of a new analgesic-AP-237". Archives Internationales de Pharmacodynamie et de Therapie. 213 (1): 28–40. PMID 1156016.
- Barlocco, D.; Cignarella, G.; Greco, G.; Novellino, E. (1993). "Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo3.2.1octane derivatives binding to the mu-opioid receptor". Journal of Computer-Aided Molecular Design. 7 (5): 557–71. doi:10.1007/bf00124362. PMID 8294946.
- Furlan D. Methyl-piperazino derivatives with analgesic activity. US 4562191
- Cignarella, G; Testa, E (1968). "2,6-Dialkylpiperazines. IV. 1-Propionyl-4-substituted cis-2,6-dimethylpiperazines structurally related to the analgetic 8-acyl-3,8-diazabicyclo3.2.1octanes". Journal of Medicinal Chemistry. 11 (3): 592–4. doi:10.1021/jm00309a039. PMID 5656502.
- Analytical Report 2-Methyl-AP-237. 19 March 2019