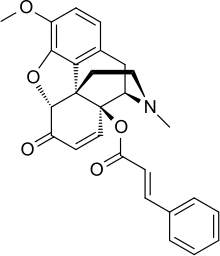
14-Cinnamoyloxycodeinone
14-Cinnamoyloxycodeinone is the most potent example in a series of opiate analgesic drugs discovered in the 1960s, with > ×100 times the potency of morphine.[1] It is a derivative of hydroxycodeinone, being the 14-cinnamate ester.[2] In another paper, Buckett assigns the potency as 177 with a range (depending on animal and test) of ×101 - ×310.[3] It may be of interest to researchers that the allyl group in this compound and in allylprodine overlay very closely.
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H25NO5 |
| Molar mass | 443.490 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- Lien, E. J.; Tong, G. L.; Srulevitch, D. B.; Dias, C. (1978). "QSAR of Narcotic Analgetic Agents" (PDF). NIDA Research Monograph. 1978 (22): 186–196. PMID 30907. Archived from the original (pdf) on 2011-10-15. Retrieved 2011-06-01.
- Buckett, W. R. (1965). "Some Pharmacological Studies With 14-Cinnamoyloxycodeinone". The Journal of Pharmacy and Pharmacology. 17 (11): 759–760. doi:10.1111/j.2042-7158.1965.tb07602.x. PMID 4379812.
- 'The relationship between analgesic activity, acute toxicity and chemical structure in esters of 14-hydroxycodeinone' W.R.Buckett J. Pharm. Pharmacol., 1964, 16, Suppl., 68T-71T
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.