Filgotinib
Filgotinib (code name GLPG0634) is a drug which is currently under investigation for the treatment of rheumatoid arthritis (RA) and Crohn's disease. It was developed by the Belgian-Dutch biotech company Galapagos NV.
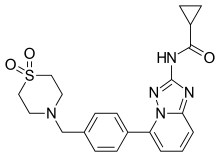 | |
| Clinical data | |
|---|---|
| Other names | GLPG0634 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 6 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H23N5O3S |
| Molar mass | 425.50402 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mechanism of action
Filgotinib is a Janus kinase inhibitor with selectivity for subtype JAK1 of this enzyme. It is considered a promising agent as it inhibits JAK1 selectively. Less selective JAK inhibitors (e.g. tofacitinib, baricitinib, and upadacitinib) are already being marketed. They show long-term efficacy in the treatment of various inflammatory diseases. However, their lack of selectivity leads to dose-limiting side effects.[1] It is thought that inhibition of all JAK isoenzymes is beneficial in rheumatoid arthritis. However, pan-JAK inhibition might also lead to unwanted side effects that might not outweigh its benefits. This is the rationale for the development of newer and more selective inhibitors like filgotinib.
The signal transmission of large numbers of proinflammatory cytokines is dependent on JAK1. Inhibition of JAK2 may also contribute to the efficacy against RA. Nonetheless it is thought that JAK2 inhibition might lead to anemia and thrombopenia by interference with erythropoietin and thrombopoietin and granulocyte-macrophage colony-stimulating factor. Therefore, one might prefer to choose a more selective JAK1 inhibitor as a primary therapeutic option. Filgotinib exerts a 30-fold selectivity for JAK1 compared to JAK2.[2] It is however still to be seen to what extent JAK2 inhibition should be avoided.
Clinical trials
The efficacy of filgotinib is currently being studied in a phase2b program (DARWIN trial 1, 2) with involvement of 886 rheumatoid arthritis patients and 180 Crohn's disease patients.
Phase 1 study
It was shown in phase 1 studies that the pharmacokinetics of filgotinib metabolism is independent of hepatic CYP450 enzymatic degradation. The drug metabolism is however mediated by carboxylesterases. There is no interference reported with the metabolism of methotrexate nor with any of the investigated transport proteins.[3]
Phase 2 study: Proof of concept (2011)
In November 2011 Galapagos released the results of their phase 2 study (identification: NCT01384422, Eudract: 2010-022953-40) in which 36 RA patients were treated who showed a suboptimal clinical response to methotrexate treatment.[4] Three groups of twelve patients were treated either with 200 mg filgotinib in a single dose, 200 mg divided in two doses or placebo. The primary end-point was the ACR20 score, which monitors improvements in the symptomatology of the patient. After the scheduled 4 weeks of treatment, 83% of the respondents showed an improved ACR20-score. Half of the treated patients showed a complete (or near complete) remission of the disease. There were no reports of anemia nor changes in lipidemia. The company stated in their press release that filgotinib is the first selective JAK1 inhibitor that shows clinical efficacy. As a result of this study, the company stated that "GLPG0634 shows one of the highest initial response rates ever reported for rheumatoid arthritis treatments".[5]
DARWIN 1 trial
The DARWIN 1 trial was a 24-week double blind placebo-controlled trial with 599 rheumatoid arthritis patients enrolled. All participants had moderate to severe RA and showed an insufficient response to standard methotrexate treatment. The trial compared three dosages of filgotinib as a once or twice per day regimen.[6] During the trial all participants remained on their methotrexate treatment. The trial completed in Feb 2015 and the results were released in July 2015.[7][8] Galapagos announced that the drug met key efficacy endpoints, showed ARC70 responses up to 39%, and maintained its safety profile.[8][9]
DARWIN 2 trial
The DARWIN 2 trial was a double blind placebo-controlled trial with 280 rheumatoid arthritis patients enrolled who show an insufficient response to standard methotrexate treatment. In contrast to the previous DARWIN 1 trial, methotrexate was discontinued. Therefore, this trial investigates filgotinib as a second-line monotherapy.[10] The recruitment of DARWIN trial 2b ended in November 2014.[11] In August 2015, Galapagos announced that the study confirmed previous results.[12]
DARWIN 3 trial
Patients who completed DARWIN 1 and 2 were eligible for DARWIN 3. On November 2017, the company announced consistent safety findings and durable activity at week 84 in the trial.[13] The estimated study completion timeframe is May 2019.[14]
FINCH Phase 3 trials
FINCH 1 looks at patients where first-line treatment with methotrexate (MTX) is not working. It compares filgotinib versus adalimumab/Humira versus a placebo.[15] FINCH 2 looks at patients where a biologic is not working. FINCH 3 looks at filgotinib as a first-line treatment unlike previous studies that investigated the drug as a second-line treatment.
FINCH 2 trial revealed patients with active rheumatoid arthritis who had an inadequate response or intolerance to one or more DMARDs, figotinib showed significance in treatment response compared with placebo.[16]
Time line
- June 2011: results of first phase 2 trial
- November 2014: initiation of DARWIN 1 and 2 trials
- July 2015: DARWIN 1 results released
- August 2015: DARWIN 2 trial results released
- September 2015: AbbVie opted out of collaboration with Galapagos
- December 2015: Galapagos signed partnership with Gilead to co-develop & co-commercialize Filgotinib for various diseases
References
- Namour, Florence; Diderichsen, Paul Matthias; Cox, Eugène; Vayssière, Béatrice; Van der Aa, Annegret; Tasset, Chantal; Van't Klooster, Gerben (2015-02-14). "Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Modeling of Filgotinib (GLPG0634), a Selective JAK1 Inhibitor, in Support of Phase IIB Dose Selection". Clin Pharmacokinet. Epub ahead of print (8): 859–874. doi:10.1007/s40262-015-0240-z. PMC 4513223. PMID 25681059.
- Van Rompaey, L; Galien, R; Van der Aar, E; Clement-Lacroix, P; Van der Aar, E; Nelles, L; Smets, B; Lepescheux, L; Cristophe, T; Conrath, K; Vandeghinste, N; Vayssiere, B; De Vos, S; Fletcher, S; Brys, R; Van’t Klooster, G; Feyen, J; Menet, C (2013-10-01). "Preclinical characterization of GLPG0634, a selective inhibitor of JAK1 for the treatment of inflammatory diseases". J. Immunol. 191 (7): 3568–3577. doi:10.4049/jimmunol.1201348. PMID 24006460.
- Florence, Namour; Julie, Desrivot; Van der Aa, Annegret; Tasset, Chantal; van 't Klooster, Gerben (2014). "Phase 1 and Phase 2 Data Confirm That GLPG0634, a Selective JAK1 Inhibitor, Has a Low Potential for Drug-Drug Interactions". Meeting Abstracts. 2014 ACR/ARHP Annual Meeting. American College of Rheumatology. 1481.
- "Safety and Preliminary Efficacy of GLPG0634 in Methotrexate-refractory Active Rheumatoid Arthritis Patients".
- "Galapagos' GLPG0634 shows excellent efficacy and safety in rheumatoid arthritis Phase II study" (PDF) (Press release). Retrieved 2015-02-26.
- "Dose-finding Study of GLPG0634 as add-on to Methotrexate in Active Rheumatoid Arthritis Patients (DARWIN1)".
- "Galapagos reports that the last patient in DARWIN 1 has completed 12 weeks of treatment" (PDF) (Press release). Retrieved 2015-02-26.
- "Galapagos' selective JAK1 inhibitor filgotinib meets key efficacy endpoints, shows ACR70 responses up to 39%, and maintains safety profile after 24 weeks of treatment in DARWIN 1 Phase 2B study".
- Westhovens, R.; Taylor, P. C.; Alten, R.; Pavlova, D.; Enríquez-Sosa, F.; Mazur, M.; Greenwald, M.; Van der Aa, A.; Vanhoutte, F. (June 2017). "Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1)". Annals of the Rheumatic Diseases. 76 (6): 998–1008. doi:10.1136/annrheumdis-2016-210104. ISSN 1468-2060. PMID 27993829.
- "Galapagos completes recruitment for Darwin 1 study with GLPG0634 (filgotinib) in RA" (Press release). Galapagos NV. Retrieved 2015-02-26 – via GlobeNewswire.
- "Galapagos completes recruitment for Darwin 2 monotherapy study with GLPG0634 (filgotinib) in RA" (Press release). Galapagos NV. Retrieved 2015-02-26 – via GlobeNewswire.
- "DARWIN 2 24-week monotherapy data in RA confirm previous results and support best-in-class potential for filgotinib".
- "Consistent safety findings and durable activity with filgotinib treatment of rheumatoid arthritis patients up to week 84 in DARWIN 3 study".
- "Long-term Follow-up Study of GLPG0634 in Active Rheumatoid Arthritis Patients - Full Text View - ClinicalTrials.gov". Retrieved 2018-01-08.
- "Filgotinib program in RA - Galapagos Annual Report 2016". reports.glpg.com. Retrieved 2018-01-08.
- Takeuchi, Tsutomu; Walker, David; Vlam, Kurt de; Sundy, John S.; Tasset, Chantal; Guo, Ying; Gao, Jie; Matzkies, Franziska; Bartok, Beatrix (2019-07-23). "Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial". JAMA. 322 (4): 315–325. doi:10.1001/jama.2019.9055. ISSN 0098-7484.
- "Study to Evaluate the Testicular Safety of Filgotinib in Adult Males With Moderately to Severely Active Ulcerative Colitis - Full Text View - ClinicalTrials.gov". Retrieved 2018-01-08.
- "Galapagos, Gilead include high dose in PhIII RA trial after talk with FDA | FierceBiotech". www.fiercebiotech.com. Retrieved 2018-01-08.