Baricitinib
Baricitinib (trade name Olumiant) is a drug for the treatment of rheumatoid arthritis (RA), being developed by Incyte and Eli Lilly.[1] It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.[2] The drug is approved in Europe.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Olumiant, Baricinix |
| Other names | INCB28050, LY3009104 |
| License data | |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 79% |
| Protein binding | 50% |
| Metabolism | CYP3A4 (<10%) |
| Elimination half-life | 12.5 hours |
| Excretion | 75% urine, 20% faeces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.219.080 |
| Chemical and physical data | |
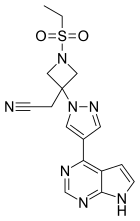
| Formula | C16H17N7O2S |
| Molar mass | 371.42 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Approvals and indications
In February 2017 Baricitinib was approved for use in the EU as a second-line therapy for moderate to severe active rheumatoid arthritis in adults, either alone or in combination with methotrexate.[4]
Baricitinib received a CRL from the FDA in April, 2017. The letter indicated that the FDA was unable to approve the application in its current form. Specifically, the FDA indicated that additional clinical data are needed to determine the most appropriate doses and that additional data is necessary to further characterize safety concerns across treatment arms.
On 23 April, 2018, an FDA Advisory Committee recommended approval of baricitinib 2mg for the treatment of rheumatoid arthritis but did not recommend the 4mg dose, citing serious adverse events.[5] On 31 May 2018 FDA approved Barictinib for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies.[6]
Contraindications
During pregnancy, the use of baricitinib is contraindicated.[4]
Side effects
In studies, upper respiratory tract infections and high blood cholesterol levels (hypercholesterolemia) occurred in more than 10% of patients. Less common side effects included other infections such as herpes zoster, herpes simplex, urinary tract infections, and gastroenteritis.[4]
Interactions
Being metabolised only to a small extent, the substance has a low potential for interactions. In studies, inhibitors of the liver enzymes CYP3A4, CYP2C19 and CYP2C9, as well as the CYP3A4 inducer rifampicin, had no relevant influence on baricitinib concentrations in the bloodstream. While it blocks a number of transporter proteins in vitro, clinically relevant interactions via this mechanism are considered very unlikely, except perhaps for the cation transporter SLC22A1 (OCT1).[4]
An additive effect with other immunosuppressants cannot be excluded.[4]
Pharmacology
Mechanism of action
Baricitinib reversibly inhibits Janus kinase 1 with a half maximal inhibitory concentration (IC50) of 5.9 nM and Janus kinase 2 with an IC50 of 5.7 nM. Tyrosine kinase 2, which belongs to the same enzyme family, is affected less (IC50 = 53 nM), and Janus kinase 3 even much less (IC50 > 400 nM). Via a signal transduction pathway involving STAT proteins, this ultimately modulates gene expression in immunological cells.[4]
Pharmacokinetics
The substance is quickly absorbed from the gut with an absolute bioavailability of 79%. It reaches highest blood plasma levels after about an hour, in different individuals ranging from 0.5 to 3 hours. Food intake has no relevant influence on the drug's pharmacokinetics. 50% of the circulating baricitinib are bound to blood plasma proteins.[4]
Less than 10% of the substance is metabolised to four different oxidation products by CYP3A4; the rest is left unchanged. Elimination half-life is 12.5 hours on average. About 75% is eliminated via the urine, and 20% via the faeces.[4]
History
Clinical trials
As of August 2016 31 clinical trials had been registered for baricitinib of which 24 had completed,[7] and 4 of 6 phase 3 trials had completed.[8]
Approval process
In January 2016, Eli Lilly submitted a new drug application to the US FDA for the approval of baricitinib to treat moderately-to-severely active rheumatoid arthritis.[1]
In December 2016, the European Committee for Medicinal Products for Human Use (CHMP) recommended the approval of baricitinib as a therapy for RA.[2] European approval was granted in February 2017.[3]
Despite widespread expectations that the FDA would approve baricitinib for RA,[9] in April 2017, the FDA issued a rejection, citing concerns about dosing and safety.[10][11]
In April 2018, and FDA Advisory Committee recommended approval for baricitinib 2mg for the treatment of rheumatoid arthritis.[5]
Brand names
In Bangladesh it is under the trade name Baricinix among others.
See also
Other JAK inhibitors include tofacitinib, currently approved for the treatment of RA,[12] and ruxolitinib.[13]
References
- "Lilly and Incyte Announce Submission of NDA to FDA for Oral Once-Daily Baricitinib for Treatment of Moderate-to-Severe Rheumatoid Arthritis". Drugs.com. 19 January 2016.
- "Summary of opinion for Olumiant" (PDF). European Medicines Agency. 15 December 2016.
- "Olumiant: EPAR – Product Information" (PDF). European Medicines Agency. 13 February 2017.
- FDA Briefing Document —Arthritis Advisory Committee Meeting
- baricitinib trials
- baricitinib phase 3 trials
- Carroll, J (13 April 2017). "We don't know when (exactly) Lilly will announce the FDA's baricitinib decision, but watch out for the looming pricing squabble". Endpoints News.
- Ramsey, L (17 April 2017). "The FDA shot down a new rheumatoid arthritis drug — and the companies that make the drug are tumbling". Business Insider.
- Grant, Ch (14 April 2017). "Surprise FDA Rejection Will Sting This Biotech". The Wall Street Journal.
- "FDA approves Xeljanz for rheumatoid arthritis". 6 November 2012.
- Mesa RA (2010). "Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis". IDrugs. 13 (6): 394–403. PMID 20506062.