Upadacitinib
Upadacitinib (trade name Rinvoq; code name ABT-494) is a drug for treatment of rheumatoid arthritis. Approved by U.S FDA on 16 August 2019.[2] It was developed by the biotech company AbbVie.
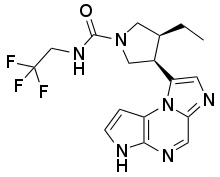 | |
| Clinical data | |
|---|---|
| Trade names | Rinvoq |
| Other names | ABT-494 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP3A major, CYP2D6 minor) [1] |
| Elimination half-life | 6-15 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H19F3N6O |
| Molar mass | 380.375 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mechanism of action
The Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases whose function is to transduce cytokine-mediated signals via the JAK-STAT pathway. There are four JAK subtypes, each of which has overlapping receptor responsibilities. Inhibitors of this enzyme family (jakinibs) have shown efficacy in treating certain inflammatory and autoimmune diseases such as rheumatoid arthritis and Crohn's disease. However, the first generation of these drugs, tofacitinib and ruxolitinib, lacked subtype selectivity, affecting JAK1/JAK3 and JAK1/JAK2 respectively. This has led to dose-limiting side effects in this otherwise promising class of drugs.[3][4] Upadacitinib is a second generation Janus kinase inhibitor that is selective for the JAK1 subtype of this enzyme over the JAK2 (74-fold), JAK3 (58-fold) and tyrosine kinase 2 subtypes.[5]
Clinical trials
Phase I studies
A phase I study revealed that upadacitinib followed a bi-exponential disposition with a terminal half-life of 6–16 hours.[1] There was no significant accumulation over the dose range of 3–36 mg per day. No interaction was found in rheumatoid arthritis patients taking methotrexate. The most common adverse event was headache but its incidence was similar to that when taking placebo (15.6% for upadacitinib vs. 16.7% for placebo). An investigation into absorption and metabolism found that dosing after a high-fat meal had no effect on upadacitinib total drug exposure over time (area under the curve or AUC).[6] Inhibition of CYP3A by ketoconazole increased total AUC, indicating the importance of this metabolic route.
Phase II studies
Two phase IIb studies were initiated to study the efficacy and safety of upadacitinib in patients with rheumatoid arthritis and one phase II study was initiated in patients with Crohn's disease.
BALANCE I
In the first study, 276 rheumatoid arthritis patients were recruited who had previously experienced inadequate response to anti–tumor necrosis factor (TNF) therapy and were currently on a stable dose of methotrexate.[7] Patients were randomized to receive 3, 6, 12, or 18 mg twice daily or placebo. The primary endpoint was a 20% improvement in symptoms according to the American College of Rheumatology improvement criteria (ACR20). At the completion of the study it was found that response rates were significantly higher in those receiving upadacitinib versus in those receiving placebo alone (36–42% and 22– 26%, respectively). Adverse events included headache, nausea, and infection but no infections were serious.
BALANCE II
In the second phase IIb study, 300 rheumatoid arthritis patients were recruited who have had an inadequate response to methotrexate.[8] Patients were randomized to receive 3, 6, 12, or 18 mg twice daily or placebo. The primary endpoint was a 20% improvement in symptoms according to the American College of Rheumatology improvement criteria (ACR20). At the completion of the study it was found that response rates were significantly higher in those receiving upadacitinib versus in those receiving placebo alone. (62%, 68%, 80%, 64%, and 76% for the 3, 6, 12, 18, and 24 mg doses, respectively) than with placebo (46%). Improvement in symptoms was rapid, with significant changes in disease scores by week 2. Adverse events were mild with infection being the most serious. One case of community-acquired pneumonia occurred at 12 mg.
CELEST
In this 16-week study, 220 patients were recruited with moderately to severely active Crohn's disease. Participants must have also experienced an inadequate response to or intolerance to Immunotherapy or TNF inhibitors.[9][10] Patients were randomized to therapy with upadacitinib at 3, 6, 12, 24 mg twice daily or 24 mg once daily for 16 weeks or placebo, followed by blinded extension therapy for 36 weeks. The co-primary endpoints were the proportion of patients who achieved clinical remission (soft stool frequency or daily abdominal pain score) at week 16 and endoscopic remission at week 12 or 16. Secondary endpoints included significant clinical response (≥30% reduction in symptoms) at week 16 and endoscopic response (≥25% decrease in symptoms) at week 12 or 16. At 16 weeks 22% of patients taking the 24 mg twice daily dose achieved endoscopic remission with upadacitinib compared to 0% of patients taking placebo. 27% of patients taking the 6 mg twice daily dose achieved clinical remission compared to 11% of patients taking placebo. Adverse events did not appear to be dose-related. A single case of non-melanoma skin cancer was reported in the 24 mg twice daily group.
Phase III studies
Abbvie has planned a total of six phase III trials that will evaluate over 4,000 patients with moderate to severe rheumatoid arthritis.[11] Two phase III trials are planned studying patients with psoriatic arthritis and one in patients with ulcerative colitis.
See also
Filgotinib is also a selective inhibitor of JAK1.
References
- Mohamed, Mohamed-Eslam F.; Camp, Heidi S.; Jiang, Ping; Padley, Robert J.; Asatryan, Armen; Othman, Ahmed A. (December 2016). "Pharmacokinetics, Safety and Tolerability of ABT-494, a Novel Selective JAK 1 Inhibitor, in Healthy Volunteers and Subjects with Rheumatoid Arthritis". Clinical Pharmacokinetics. 55 (12): 1547–1558. doi:10.1007/s40262-016-0419-y. ISSN 1179-1926. PMID 27272171.
- "AbbVie Receives FDA Approval of RINVOQ™ (upadacitinib), an Oral JAK Inhibitor For The Treatment of Moderate to Severe Rheumatoid Arthritis". news.abbvie.com. Retrieved 16 August 2019.
- Fleischmann, Roy (May 2012). "Novel small-molecular therapeutics for rheumatoid arthritis". Current Opinion in Rheumatology. 24 (3): 335–341. doi:10.1097/BOR.0b013e32835190ef. ISSN 1531-6963. PMID 22357358.
- Riese, Richard J.; Krishnaswami, Sriram; Kremer, Joel (August 2010). "Inhibition of JAK kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes". Best Practice & Research. Clinical Rheumatology. 24 (4): 513–526. doi:10.1016/j.berh.2010.02.003. ISSN 1532-1770. PMID 20732649.
- "Characterization of ABT-494, a Second Generation Jak1 Selective Inhibitor". ACR Meeting Abstracts. Retrieved 2017-05-21.
- Mohamed, Mohamed-Eslam F.; Jungerwirth, Steven; Asatryan, Armen; Jiang, Ping; Othman, Ahmed A. (2017-05-14). "Assessment of effect of CYP3A Inhibition, CYP Induction, OATP1B Inhibition, and High-Fat Meal on Pharmacokinetics of the JAK1 inhibitor Upadacitinib". British Journal of Clinical Pharmacology. doi:10.1111/bcp.13329. ISSN 1365-2125. PMC 5595971. PMID 28503781.
- Kremer, Joel M.; Emery, Paul; Camp, Heidi S.; Friedman, Alan; Wang, Li; Othman, Ahmed A.; Khan, Nasser; Pangan, Aileen L.; Jungerwirth, Steven; Keystone, Edward C. (December 2016). "A Phase IIb Study of ABT‐494, a Selective JAK‐1 Inhibitor, in Patients With Rheumatoid Arthritis and an Inadequate Response to Anti–Tumor Necrosis Factor Therapy". Arthritis & Rheumatology. 68 (12): 2867–2877. doi:10.1002/art.39801. ISSN 2326-5191. PMC 5132116. PMID 27389975.
- Genovese, Mark C.; Smolen, Josef S.; Weinblatt, Michael E.; Burmester, Gerd R.; Meerwein, Sebastian; Camp, Heidi S.; Wang, Li; Othman, Ahmed A.; Khan, Nasser; Pangan, Aileen L.; Jungerwirth, Steven (December 2016). "Efficacy and Safety of ABT‐494, a Selective JAK‐1 Inhibitor, in a Phase IIb Study in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate". Arthritis & Rheumatology. 68 (12): 2857–2866. doi:10.1002/art.39808. ISSN 2326-5191. PMC 5132065. PMID 27390150.
- "A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of ABT-494 for the Induction of Symptomatic and Endoscopic Remission in Subjects With Moderately to Severely Active Crohn's Disease Who Have Inadequately Responded to or Are Intolerant to Immunomodulators or Anti-TNF Therapy - Full Text View - ClinicalTrials.gov". Retrieved 2017-05-22.
- "AbbVie Announces Positive Phase 2 Study Results for Upadacitinib (ABT-494), an Investigational JAK1-Selective Inhibitor, in Crohn's Disease". Retrieved 2017-05-22.
- Phase 3 upadacitinib trials