EVT-101
EVT-101, also known as ENS-101, is an experimental medication which originated from Roche and is under development by Evotec AG for the treatment of major depressive disorder.[1] It acts as a selective NMDA receptor subunit 2B (NR2B) antagonist.[1][2] As of November 2017, EVT-101 is in phase II clinical trials for major depressive disorder, although no recent reports of development have been identified.[1] The drug was first claimed by Roche in 2002.[3]
 | |
| Clinical data | |
|---|---|
| Other names | ENS-101 |
| Drug class | NMDA receptor antagonist |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
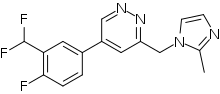
| Formula | C16H13F3N4 |
| Molar mass | 318.303 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- http://adisinsight.springer.com/drugs/800023880
- https://www.evotec.com/uploads/media_library/14/AC_SfN_Poster_2010_FINAL.pdf
- Santangelo RM, Acker TM, Zimmerman SS, Katzman BM, Strong KL, Traynelis SF, Liotta DC (2012). "Novel NMDA receptor modulators: an update". Expert Opin Ther Pat. 22 (11): 1337–52. doi:10.1517/13543776.2012.728587. PMC 3677696. PMID 23009122.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.