STDs in Women and Infants
Public Health Impact
Women and their infants are uniquely vulnerable to the consequences of sexually transmitted infections (STI). While individual-level determinants, including high-risk behaviors, contribute to disease transmission and acquisition risk, it is widely accepted that social barriers to STD prevention and control efforts also contribute to infectious disease prevalence. A woman’s relationship status with her male partner, such as the concurrency of the relationship, may be an important predictor of her sexual health.1–3 In addition to social factors such as poverty and lack of access to quality STD services, homelessness or unstable housing may influence a woman’s sexual risk.4 For some women, maintaining the relationship with a partner may take a higher priority than STD risk reduction,5 health, as well as the health of her unborn baby.6,7 A woman can also be placed at risk for STIs through her partner’s sexual encounter with an infected partner. Consequently, even a woman who has only partner may be obliged to practice safer sex, such as using condoms.8
Chlamydia and gonorrhea disproportionately affect women because early infection may be asymptomatic and, if untreated, infection may ascend to the upper reproductive tract resulting in pelvic inflammatory disease (PID). Data from prospective studies suggest that about 15% of untreated chlamydial infections progress to clinically diagnosed PID and the risk with untreated gonococcal infection may be even higher.9–11 PID is a major inflammation and damage to the fallopian tubes, elevating the risk of infertility and ectopic pregnancy. Tubal factor infertility ranks among the most common causes of infertility, accounting for 30% of female infertility in the United States,12 and much of this damage results from previous episodes of PID.13
An important public health measure for preventing PID, and ultimately tubal factor infertility, is through the prevention and control of C. trachomatis and N. gonorrhoeae. Strategies to improve the early detection and treatment of chlamydia and gonorrhea, as demonstrated in a number of randomized controlled trials,14 has been shown to reduce a woman’s risk for PID and ultimately protect the fertility of women.
Human papillomavirus (HPV) infections are highly prevalent in the United States, especially among young sexually active adults. Although most HPV infections in women appear to be transient and may not result in clinically significant sequelae, high-risk HPVtype infections can cause abnormal changes in the uterine cervical epithelium, which are detected by cytological examination of Papanicolaou (Pap) smears. Persistent
high-risk HPV-type infections may lead to cervical cancer precursors, which, if undetected can result in cancer. Other low-risk HPV-type infections can cause genital warts, low-grade Pap smear abnormalities, and, rarely, recurrent respiratory papillomatosis in infants born to infected mothers.15 For more information on adolescent and adult HPV infections, see Other STDs.
Impact on Maternal and Fetal Outcomes
Similar to non-pregnant women, a high proportion of pregnant women with chlamydial and gonococcal infections are asymptomatic. Documented sequelae of untreated infections in pregnancy include stillbirth, premature delivery, premature rupture of the membranes, and low birth weight. Maternal infection can also affect the infant, leading to conjunctivitis infections (termed ophthalmia neonatorum in the first four weeks of life), and, in the case of C. trachomatis, pneumonia. Although topical prophylaxis of infants at delivery is effective for prevention of gonococcal ophthalmia neonatorum, prevention of neonatal pneumonia requires prenatal detection and treatment. The clinical presentation of conjunctivitis can be variable and these infections are especially important to treat promptly, as they can lead to visual impairment.16
Syphilis has long been known to be an important risk factor for adverse pregnancy outcomes. The consequences of untreated maternal infection include fetal death, preterm birth, and also congenital infection in a proportion of surviving infants resulting in both physical and mental developmental disabilities. Most cases of congenital syphilis are easily preventable if women are screened for syphilis and treated early during prenatal care.17
Genital infections with herpes simplex virus (HSV) are extremely common, can cause painful outbreaks, and can have serious consequences for pregnant women and their infants.18 Neonatal herpes can be a severe illness presenting with pulmonary disease, seizures, fever, and a high case fatality rate following contact with infected cervical or vaginal secretions during delivery. Risk of transmission to the infant is greatest when the mother has a first-episode primary genital infection during pregnancy, especially if she acquires infection towards the end of her pregnancy.19,20
Observations
Pelvic Inflammatory Disease
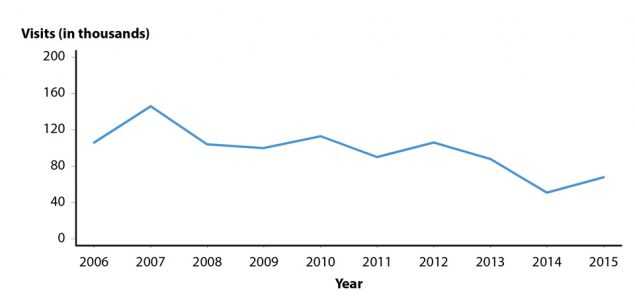
Figure A. Pelvic Inflammatory Disease — Initial Visits to Physicians’ Offices Among Women Aged 15–44 Years, United States, 2006–2015
Accurate estimates of PID and tubal factor infertility resulting from chlamydial and gonococcal infections are difficult to obtain, in part because definitive diagnoses of these conditions can be complex. Published data suggest overall declining United States rates of women diagnosed with PID in both hospital and ambulatory settings.21–23 The National Disease and Therapeutic Index (NDTI; see Section A2.5 in the Appendix) provides estimates of initial visits to office based, private physicians for PID. NDTI estimated that from 2006–2015 the number of initial visits to such physicians for PID among women aged 15–44 years have decreased by 35.8% from 106,000 to 68,000 visits (Figure A). The 2016 NDTI data were not obtained in time to include them in this report. Several factors may be contributing to declining PID rates, including increases in chlamydia and gonorrhea screening coverage, more sensitive diagnostic technologies, and availability of single-dose therapies that increase adherence to treatment.22–24 While PID is declining nationally, it still causes an enormous amount of unnecessary and expensive morbidity.
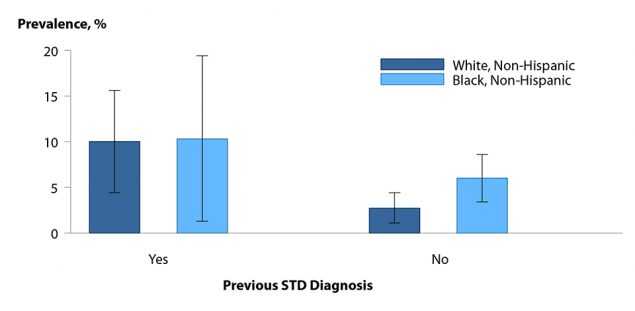
Figure B. Pelvic Inflammatory Disease — Lifetime Prevalence Among Sexually Experienced Women Aged 18–44 Years by Race/Ethnicity and Previous STI Diagnosis, National Health and Nutrition Examination Survey (NHANES), 2013–2014
Differences in self-reported lifetime diagnosis of PID by race/ethnicity in reproductive age women have been observed in earlier research.25 Data from the 2013–2014 cycle of the National Health and Nutrition Examination Survey (NHANES) indicates that non-Hispanic Black and non-Hispanic White women reporting a previous STI diagnosis had nearly equal self-reported lifetime PID prevalence (10.0% vs. 10.3%). However, the lifetime prevalence of PID among non-Hispanic Black women was 2.2 times that among non-Hispanic White women if no previous STI was diagnosed (6.0% vs. 2.7%) (Figure B). These findings suggest that PID is associated with previous STI diagnoses and it is therefore important for physicians to screen female patients for chlamydia and gonorrhea to reduce the incidence of PID. The racial disparities observed in PID diagnoses are consistent with the marked racial disparities observed for chlamydia and gonorrhea. However, because of the subjective methods by which PID is diagnosed, racial disparity data should be interpreted with caution.
Ectopic Pregnancy
Ectopic pregnancy is a potentially life-threatening condition that requires prompt evaluation and treatment. Up until the early 1990’s, a primary data source used to estimate the incidence of ectopic pregnancy was the National Hospital Discharge Survey, a sample of inpatient discharge records from select hospitals. However, the ability to ascertain the number of ectopic pregnancies occurring in the United States has been affected by a shift in clinical management from an inpatient to an outpatient event, making national surveillance data sources unreliable. As a result, alternative surveillance methods, including data from large administrative claims,26,27 are needed to evaluate trends and assess the continued public health burden of this condition. Data from MarketScan Commercial Claims and Encounters Database, a large administrative claims database of United States commercial health plans, indicate that rates of ectopic pregnancy increased with age and that trends in the rate of ectopic pregnancy among women with live births aged 15–44 years during the period of 2004–2014 were relatively stable across all age groups. However, a potential deflection in the trend is observed from 2014–2015 across all 5-year age groups, for which additional years of data are needed to appropriately assess (Figure C). As in previous years, in 2015, rates of ectopic pregnancy were highest among women in the 35–39 and 40–44 year age groups.
Chlamydia
Chlamydial infections in women are usually asymptomatic and screening is necessary to identify most infections. Routine chlamydia screening of sexually-active young women has been recommended by the CDC since 1993.28 Rates of reported cases of chlamydia among women increased steadily from the early 1990s, likely reflecting expanded screening coverage and use of more sensitive diagnostic tests (Figure 1, Table 1). During 2011–2013, chlamydia case rates decreased from 643.4 to 619.0 cases per 100,000 females and then increased 6.2% over the next 3 years, resulting in a rate of 657.3 cases per 100,000 females in 2016 (Table 4).
Chlamydia rates are highest among young women, the population targeted for screening (Figure 5, Table 10). During 2015–2016, rates of reported chlamydia cases increased 2.8% and 0.4% among females aged 15–19 and 20–24 years, respectively. Regionally, chlamydia case rates were highest among women in the South, with a rate of 718.6 cases per 100,000 females in 2016 (Table 4). Rates of reported chlamydia cases exceeded gonorrhea case rates among women in all regions (Figures D and E, Tables 4 and 15).
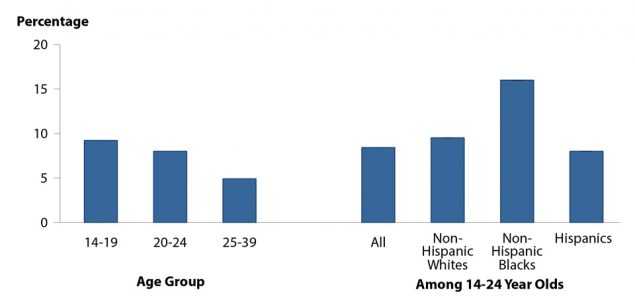
Chlamydia Positivity in Selected Populations
The STD Surveillance Network (SSuN) is an ongoing collaboration of state, county, and city health departments from 10 participating jurisdictions where demographic, clinical, and laboratory data are collected from women aged 15–44 years attending facilities that provide family planning and reproductive health services (See Section A2.2 of the Appendix). Figure F shows chlamydia testing and positivity reported only among facilities that tested more than 100 women and more than 60% of young women aged 14–24 years. In 2016, the overall positivity of chlamydia among women aged 14–24 years was 8.4%, but for women 14–19 years of age, chlamydia positivity was 9.4%. For women between the ages of 14–24 years, chlamydia positivity among non-Hispanic Blacks was almost twice that of non-Hispanic Whites or Hispanics.
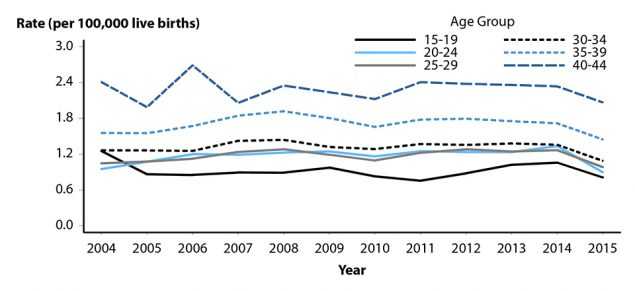
Figure C. Ectopic Pregnancy — Rates Among Commercially Insured Women with Live Births Aged 15–44 Years by Age Group, 2004–2015
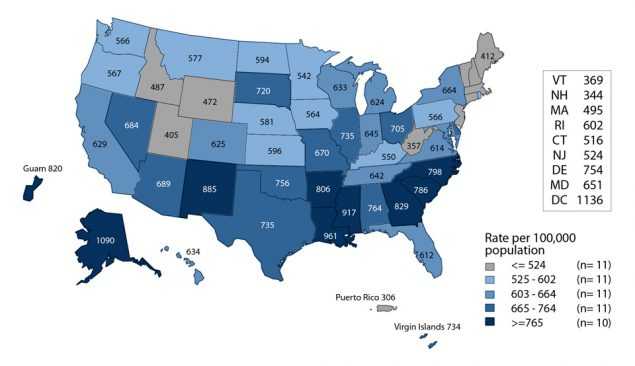
Figure D. Chlamydia — Rates of Reported Cases Among Women by State, United States and Outlying Areas, 2016
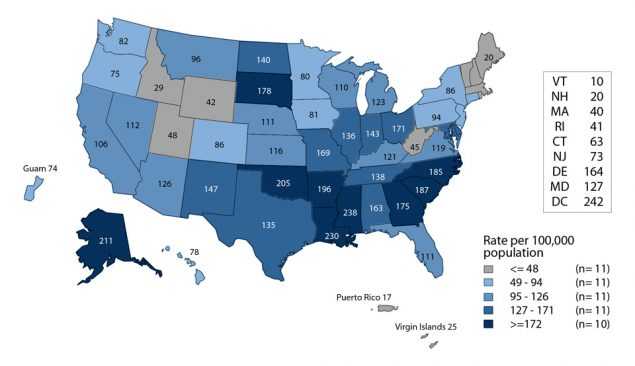
Figure E. Gonorrhea — Rates of Reported Cases Among Women by State, United States and Outlying Areas, 2016
Gonorrhea
Like chlamydia, gonorrhea is often asymptomatic in women. Therefore, gonorrhea screening is an important strategy for the identification of gonorrhea among women. Large-scale screening programs for gonorrhea in women began in the 1970s. After an initial increase in cases detected through screening, rates of reported gonorrhea cases for both women and men declined steadily throughout the 1980s and early 1990s, and then declined more gradually in the late 1990s and the 2000s. However, more recently, there have been increases in overall cases. (Figure 12, Table 1).
After reaching a 40-year low in 2009 (104.5 cases per 100,000 females), the rate of reported cases of gonorrhea for women increased slightly each year during 2009–2011, and then decreased during 2012–2014 (Figure 13). During 2015–2016, the gonorrhea rate among women increased 13.8% to 121.0 cases per 100,000 females (Figure 13, Table 15).
The gonorrhea case rate among women was slightly higher than the rate among men during 2007–2012; however, the rate among men was higher than the rate among women during 2013–2016 (Figure 13, Tables 15 and 16). During 2012–2016, gonorrhea rates among women were highest among those aged 15–24 years (Figure 17, Table 21). For women in this age group, rates were highest among 19-year olds in 2016 (736.2 cases per 100,000 females) (Table 23).
Neonatal Conjunctivitis
During 2010–2015, 563 chlamydia or gonorrhea cases among infants aged <1 year with a specimen source of either ‘eye’ or ‘conjunctiva’ (conjunctivitis infections) were reported to CDC.29 During 2010–2014, the overall reported rate of chlamydial conjunctivitis in infants decreased by 40.7% (2.7 to 1.6 cases per 100,000 live births), followed by an increase of 31.2% during 2014–2015 to 2.1 cases per 100,000 live births (Figure G). The rate of gonococcal conjunctivitis in infants remained relatively constant and low during 2010–2015, at 0.2 cases or less per 100,000 live births each year. The rate of reported cases is heavily influenced by the completeness of reported data on specimen source. Of all cases reported to CDC of chlamydia or gonorrhea in infants aged <1 year during 2010–2015 (n=3,703), nearly 85% did not have a specimen source of either ‘eye’ or ‘conjunctiva’; of those, 52% had a specimen source of ‘unknown’ (40.1%), ‘other-not specified’ (8.7%), or was missing (3.2%). When evaluating rates including these cases, the rate of chlamydia and gonorrhea infections follows similar trends but is higher in all years, indicating potential missed cases for surveillance (Figure G).
Congenital Syphilis
Trends in congenital syphilis usually follow trends in primary and secondary (P&S) syphilis among women (Figure 44). After plateauing at a relatively low rate (0.9 cases per 100,000 females) during 2011–2013, the rate of reported P&S syphilis cases has increased each year since then. During 2015–2016, the rate among women increased 35.7% to 1.9 cases per 100,000 females (Figure 44, Table 28).
Similarly, the rate of reported congenital syphilis cases has increased each year since 2012 (Figure 44, Table 1). In 2016, there were 628 reported cases of congenital syphilis and the national congenital syphilis rate was 15.7 cases per 100,000 live births, the highest rate reported since 1998. This increase in 2016 represents a 27.6% increase relative to 2015 and an 86.9% increase relative to 2012 (Table 41).
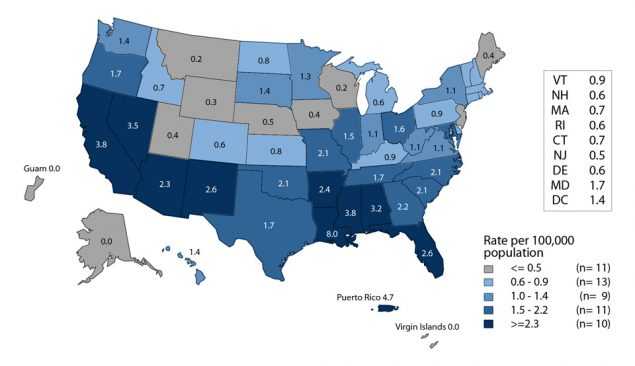
Figure H. Primary and Secondary Syphilis — Rates of Reported Cases Among Women by State, United States and Outlying Areas, 2016
In 2016, the highest rates of P&S syphilis among women and the highest rates of congenital syphilis were observed in the West and in the South (Figures H and I, Tables 28 and 41). The P&S syphilis rates among women increased in every region during 2015–2016. During 2015–2016, the largest increases in the P&S syphilis rates among women were seen in the West (58.8%), followed by the Northeast (28.6%), South (22.2%), and Midwest (20.0%). The congenital syphilis rate increased 42.1% in the Northeast, 41.4% in the West, and 23.6% in the South, and remained stable in the Midwest during 2015–2016 (Table 41).
Although most cases of congenital syphilis occur among infants whose mothers have had some prenatal care, late or limited prenatal care has been associated with congenital syphilis. Failure of health care providers to adhere to maternal syphilis screening recommendations also contributes to the occurrence of congenital syphilis.17
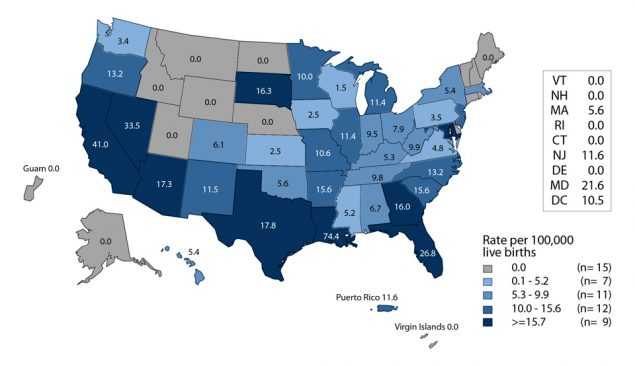
Figure I. Congenital Syphilis — Rates of Reported Cases Among Infants by Year of Birth and State, United States and Outlying Areas, 2016
Neonatal Herpes Simplex Virus
Neonatal HSV infections, although relatively rare, cause significant morbidity and mortality.18 Most neonatal HSV infections result from perinatal transmission from mother to neonate,20 but postnatal infection can occur.30 Although reporting of neonatal HSV infection is required in a few jurisdictions,31,32 it is not a nationally reportable disease.
An examination of inpatient records of infants aged 60 days or younger at admission using the Healthcare Cost and Utilization Project Kid’s Inpatient Database showed an overall incidence of 9.6 cases per 100,000 live births in 2006.33 Rates did not vary significantly by region or race/ethnicity; however prevalence was significantly higher among cases for which the expected primary payer was Medicaid (15.1 cases per 100,000 live births) compared with private insurance or managed health care (5.4 cases per 100,000 live births).
In New York City, 76 cases of neonatal HSV infection were identified through population based surveillance during a 4.5 year period (April 2006–September 2010), for an average annual incidence of 13.3 cases per 100,000 live births.34 Forty-one percent of the confirmed cases were infected with HSV type 1. A review of certificates of death or stillbirth issued in New York City during 1981–2013 identified 34 deaths due to neonatal HSV infection, or 0.82 deaths per 100,000 live births.33
For information on adolescent and adult HSV infections, see Other STDs.
References
1. Jolly DH, Mueller MP, Chen M, et al. Concurrency and other sexual risk behaviors among black young adults in a southeastern city. AIDS Educ Prev 2016; 28(1):59–76.
2. Dolwick Grieb SM, Davey-Rothwell M, Latkin CA. Concurrent sexual partnerships among urban African American high risk women with main sex partners. AIDS Behav 2012; 16(2):323–333. DOI: 10.1007/s10461-011-9954-6.
3. Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis 2008; 35(12):S13–S18.
4. Kelly J, Cohen J, Grimes B, et al. High rates of herpes simplex virus type 2 infection in homeless women: informing public health strategies. J Womens Health (Larchmt) 2016; 25(8):840–845. DOI: 10.1089/jwh.2015.5579.
5. Tschann JM, Flores E, de Groat CL, et al. Condom negotiation strategies and actual condom use among Latino youth. J Adolesc Health 2010; 47(3):254–262. DOI: 10.1016/j.jadohealth.2010.01.018.
6. Pulerwitz J, Amaro H, De Jong W, et al. Relationship power, condom use and HIV risk among women in the USA. AIDS Care 2002; 14(6):789–800.
7. McCree DH, Rompalo A. Biological and behavioral risk factors associated with STDs/HIV in women: implications for behavioral interventions. In: Aral SO, Douglas JM, Lipshutz JA, eds. Behavioral Interventions for Prevention and Control of Sexually Transmitted Diseases. New York, NY: Springer; 2007:310–324.
8. Swartzendruber A, Zenilman JM, Niccolai LM, et al. It takes 2: partner attributes associated with sexually transmitted infections among adolescents. STD 2013; 40(5):372–378.
9. Haggerty CL, Gottlieb SL, Taylor BD, et al. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201:S134–S155.
10. Oakeschott, P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010; 340:c1642.
11. Price MJ, Ades AE, De Angelis D, et al. Risk of pelvic inflammatory following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol 2013; 178(3):484–492.
12. Tsevat DG, Wiesenfeld HC, Parks C, et al. Sexually transmitted diseases and infertility. Am J Obstet Gynecol 2017; 216(1):1–9.
13. Ness RB, Trautmann G, Richter HE, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease: A randomized trial. Obste Gyneco 2005; 106(3):573–580.
14. Gottlieb SL, Xu F, Brunham RC. Screening and treating chlamydia genital infections to prevent PID: interpretation of findings from randomized controlled trials. Sex Transm Dis 2013; 40(2):97–102.
15. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63(RR05):1–30.
16. Kohlhoff, SA, Hammerschlag, MR. Gonococcal and chlamydial infections in infants and children. In: Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw Hill; 2007:1613–1627.
17. Centers for Disease Control and Prevention. Congenital syphilis — United States, 2003–2008. MMWR Morb Mortal Wkly Rep 2010; 59(14):413–417.
18. Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol 2007; 31(1):19–25.
19. Kimberlin DW. The scarlet H. J Infect Dis 2014; 209(3):315–317.
20. Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med 2009; 361(14):1376–1385.
21. Sutton MY, Sternberg M, Zaidi A, et al. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sex Transm Dis 2005; 32(12):778–784.
22. Bohm MK, Newman L, Satterwhite CL, et al. Pelvic inflammatory disease among privately insured women, United States, 2001–2005. Sex Transm Dis 2010; 37(3):131–136.
23. Whiteman MK, Kuklina E, Jamieson DJ, et al. Inpatient hospitalization for gynecologic disorders in the United States. Am J Obstet Gynecol 2010; 202(6):541.e1–6.
24. Owusu Edusei K Jr, Bohm MK, Chesson HW, et al. Chlamydia screening and pelvic inflammatory disease: Insights from exploratory time-series analyses. Am J Prev Med 2010; 38(6):652–657.
25. Leichliter JS., Chandra A., Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive aged women in the United States, 1995 and 2006–2010. Sex Transm Dis 2013; 40(5):413–418.
26. Trabert B, Holt VL, Yu O, et al. Population-Based ectopic pregnancy trends, 1993–2007. Am J Prev Med 2011; 40(5):556–560.
27. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol 2010; 115(3):495–502.
28. Centers for Disease Control and Prevention. Recommendations for the prevention and management of Chlamydia trachomatis infections, 1993. MMWR Morb Mortal Wkly Rep 1993; 42(RR-12):1–39.
29. Kreisel, K, Weston, E, Braxton, et al. Keeping an eye on chlamydia and gonorrhea conjunctivitis in infants in the United States, 2010–2015. Sex Transm Dis 2017; 44(6): 356–358.
30. Centers for Disease Control and Prevention. Neonatal herpes simplex virus infection following Jewish ritual circumcisions that included direct orogenital suction — New York City, 2000–2011. MMWR Morb Mortal Wkly Rep 2012; 61(22):405–409.
31. Dinh T-H, Dunne EF, Markowitz LE, et al. Assessing neonatal herpes reporting in the United States, 2000–2005. Sex Transm Dis 2008; 35(1):19–21.
32. Handel S, Klingler EJ, Washburn K, et al. Population-based surveillance for neonatal herpes in New York City, April 2006–September 2010. Sex Transm Dis 2011; 38(8):705–711.
33. Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics 2011; 127(1):e1–8.
34. Sampath A, Maduro G, Schillinger JA. Infant deaths due to herpes simplex virus, congenital syphilis, and HIV in New York City. Pediatrics 2016; 137(4):e20152387.
- Page last reviewed: September 26, 2017
- Page last updated: September 26, 2017
- Content source:


 ShareCompartir
ShareCompartir