Other Sexually Transmitted Diseases
Chancroid
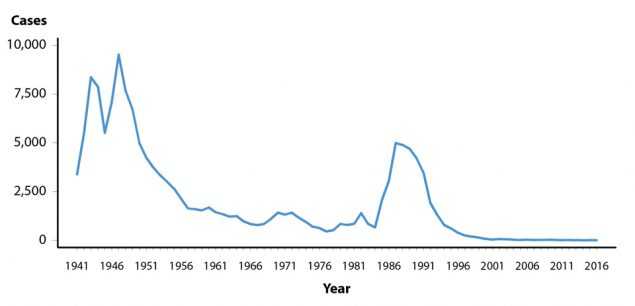
Chancroid is caused by infection with the bacterium Haemophilus ducreyi. Clinical manifestations include genital ulcers and inguinal lymphadenopathy or buboes.1 Reported cases of chancroid declined steadily between 1987 and 2001 (Figure 45, Table 1). Since then, the number of reported cases has fluctuated somewhat, while still appearing to decline overall. In 2016, a total of 7 cases of chancroid were reported in the United States. Six states reported one or more cases of chancroid in 2016 (Table 43).
Although the overall decline in reported chancroid cases most likely reflects a decline in the incidence of this disease, these data should be interpreted with caution because Haemophilus ducreyi is difficult to culture; as a result, this condition may be substantially underdiagnosed.2,3
Human Papillomavirus
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States.4 Over 40 distinct HPV types can infect the genital tract;5 although most infections are asymptomatic and appear to resolve spontaneously within a few years,6 prevalence of genital infection with any HPV type was 42.5% among United States adults aged 18–59 years during 2013–2014.7 Persistent infection with some HPV types can cause cancer and genital warts. HPV types 16 and 18 account for approximately 66% of cervical cancers in the United States,8 and approximately 25% of low-grade and 50% of high-grade cervical intraepithelial lesions, or dysplasia.9,10 HPV types 6 and 11 are responsible for approximately 90% of genital warts.11,12
Quadrivalent HPV vaccine targets HPV types 6, 11, 16, and 18.11 This vaccine was licensed in the United States in mid-2006 for females13 and in late 2009 for males.14 Although a bivalent vaccine was also licensed for females,15 almost all HPV vaccine administered in the United States through late 2014 was quadrivalent.16 A 9-valent vaccine, which protects against the quadrivalent and 5 additional oncogenic HPV types (types 31, 33, 45, 52, and 58), was licensed in late 2014 for males and females.17 All HPV vaccines have been recommended for routine use in United States females aged 11–12 years, with catch-up vaccination through age 26.13,17 Since late 2011, routine use of the quadrivalent or 9-valent vaccine has been recommended for males aged 11–12, with catch-up vaccination through age 21.18 Vaccination through age 26 is recommended for gay, bisexual, and other men who have sex with men (collectively referred to as MSM)18 and persons who are immunocompromised (including those infected with HIV).17
HPV vaccine uptake in the United States remains lower than the Healthy People 2020 goal of 80% coverage.19 In 2015, a national survey found that 63% of girls aged 13–17 years had received at least 1 dose of the HPV vaccine, and 42% had received all recommended doses in the series.20 HPV vaccine uptake is lower among boys; 50% of those aged 13–17 years received at least 1 dose, but only 28% received all recommended doses.20
HPV infection is not a nationally reportable condition. Cervicovaginal prevalence of quadrivalent HPV vaccine types 6, 11, 16, and/or 18 was estimated using data for females aged 14–34 years from the National Health and Nutrition Examination Survey (NHANES; see Section A2.4 in the Appendix). Prevalence decreased significantly from the pre-vaccine era (2003–2006) to the early postvaccine era (2009–2012) in specimens from females aged 14–19 and 20–24 years, the age groups most likely to benefit from HPV vaccination (Figure 46).21 Among those aged 25–34 years, vaccine-type HPV prevalence did not differ significantly between the two time periods, and no differences were observed in the prevalence of non-quadrivalent HPV vaccine types by time period for any age group. An NHANES analysis of 2013–2014 HPV prevalence from penile swab specimens found low prevalence of quadrivalent HPV vaccine types in young males, which the authors attributed to male vaccination and/or herd protection from female vaccination.22
Health-care claims data from 9 million females aged 15–39 years in the United States with employer provided private health insurance were used to estimate annual prevalence of cytologically-detected cervical low-grade and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively) and high-grade histologically-detected cervical intraepithelial neoplasia grades 2 and 3 (CIN2+) during 2007–2014.23 Analyses were restricted to females who received cervical cancer screening in a given calendar year. Among females aged 15–19 years, LSIL prevalence over the entire period increased (Figure 47A); annual percent change during 2007–2014 was 2.0% (P=0.041). Although prevalence in those aged 15–19 years appeared to decrease somewhat during 2013–2014, additional years of data are needed to appropriately interpret this observation. LSIL prevalence in women aged 20–24 years increased during 2007–2012 (annual percent change = 3.0%, P=0.002), then declined significantly during 2012–2014 (annual percent change = -8.2%, P=0.005). Prevalence of LSIL increased significantly during 2007–2014 for women aged 25–39 years. In contrast to LSIL, prevalence of HSIL and CIN2+ decreased significantly in females aged 15–19 and 20–24 years during 2007–2014 (Figures 47B and 47C). Among those aged 15–19 years, annual percent change in HSIL prevalence during 2007–2014 was -8.3% (P<0.001), while change in CIN2+ prevalence was -19.8% (P=0.004) during 2007–2009 and -12.1% (P=0.002) during 2009–2014. For women aged 20–24 years, annual percent change in HSIL was -5.3% (P<0.001) during 2007–2014; annual percent change in CIN2+ was -6.7% (P=0.001) during 2007–2012 and -12.5% during 2012–2014 (P=0.020). No decreases in HSIL or CIN2+ prevalence were observed in women aged 25–39 years. Decreases in high-grade lesions among young women reflected their greater association with HPV types 16 and 18, compared to low-grade lesions, providing ecologic evidence of population effectiveness of HPV vaccination on clinical sequelae of infection among young, privately insured US women.
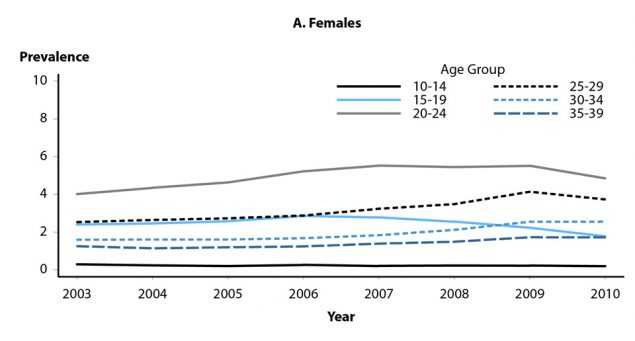
Prevalence of genital warts also has been examined using health-care claims records (Figures 48A and 48B).24 Prevalence among females aged 15–19 years was stable during 2003–2007, but then significantly declined from 2.9 per 1000 person years in 2006 to 1.8 in 2010. Among females aged 20–24 years, genital wart prevalence significantly increased during 2003–2007, then was essentially unchanged at 5.4 to 5.5 per 1000 person-years during 2007–2009; although prevalence among women in this age group appeared to decrease in 2010, more years of data are needed to determine whether this observation represents a valid inflection in trend. Prevalence in women aged 25–39 years significantly increased from 2.5 per 1000 person-years in 2003 to 3.7 in 2010, but a potential inflection in trend was also observed in this age group during 2009–2010; as with women aged 20–24, additional years of data are needed to appropriately assess this potential change in trend. Genital wart prevalence significantly increased in males aged 15–39 years during 2003–2010, although for men aged 20–24 years a potential inflection in trend was observed during 2009–2010.
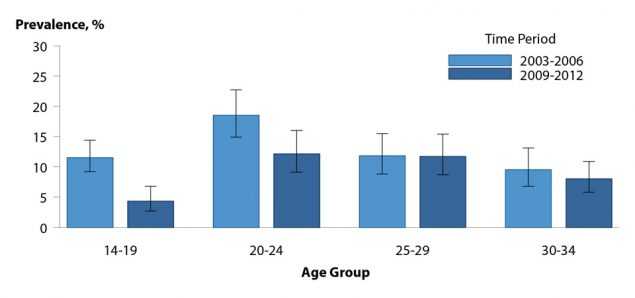
Figure 46. Human Papillomavirus — Cervicovaginal Prevalence of Types 6, 11, 16 and 18 Among Females Aged 14–34 Years by Age Group and Time Period, National Health and Nutrition Examination Survey, 2003–2006 and 2009–2012
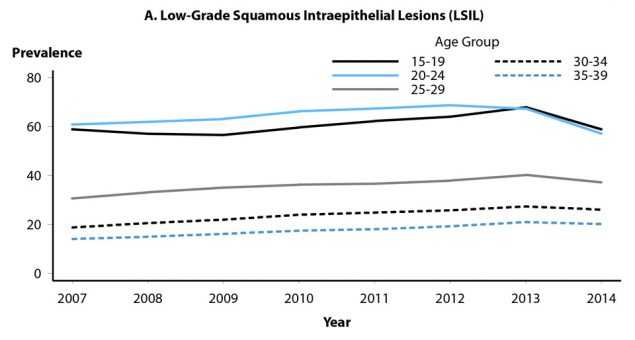
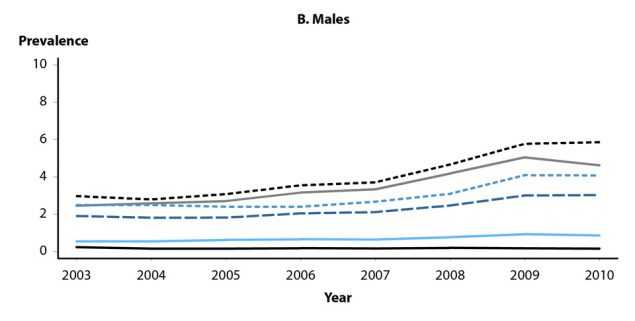
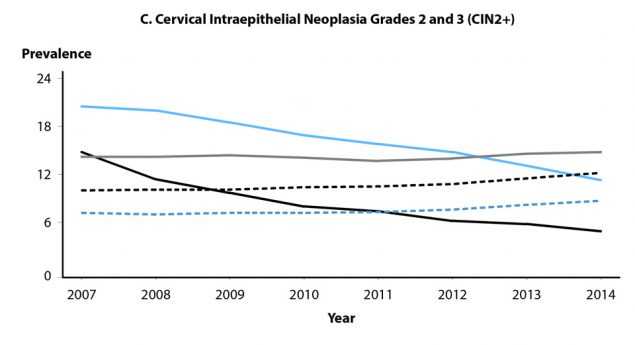
Figure 47. Cervical High-Grade Squamous Intraepithelial Lesions and Intraepithelial Neoplasia Grades 2 and 3 — Prevalence per 1000 Person-Years Among Female Participants in Private Health Plans Aged 15–39 Years, by Age Group and Year, 2007–2014
Pelvic Inflammatory Disease
For information on pelvic inflammatory disease, see Special Focus Profiles, STDs in Women and Infants.
Herpes Simplex Virus
Herpes simplex virus (HSV) is among the most prevalent of sexually transmitted infections.4,25 Although most infections are subclinical,26 clinical manifestations are characterized by recurrent, painful genital and/or anal lesions.27 Most genital HSV infections in the United States are caused by HSV type 2 (HSV-2), while HSV type 1 (HSV-1) infections are typically orolabial and acquired during childhood.26,28
HSV infection is not a nationally reportable condition. Most persons with genital HSV infection have not received a diagnosis. The overall percentage of HSV-2 seropositive NHANES participants aged 14–49 years who reported never being told by a doctor or health care professional that they had genital herpes did not change significantly between 1988–1994 and 2007–2010, and remained high (90.7% and 87.4%, respectively).29
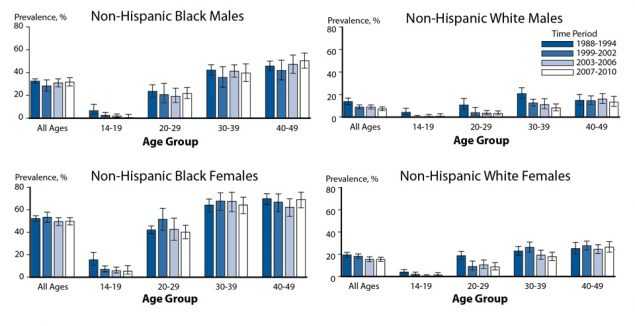
Figure 49. Herpes Simplex Virus Type 2 — Seroprevalence Among Non-Hispanic Whites and Non-Hispanic Blacks by Sex and Age Group, National Health and Nutrition Examination Survey, 1988–1994, 1999–2002, 2003–2006, and 2007–2010
NHANES data on the gender- and race/ethnicity-specific seroprevalence of HSV-2 among those aged 14–49 years were compared across survey years 1988–1994, 1999–2002, 2003–2006, and 2007–2010 (Figure 49). Overall, HSV-2 seroprevalence decreased between 1988–1994 and 2007–2010, from 21.2% to 15.5%.29 Among non-Hispanic White females, HSV-2 seroprevalence significantly decreased from 19.5% in 1988–1994 to 15.3% in 2007– 2010; HSV-2 seroprevalence remained stable among non-Hispanic Black females, from 52.5% during 1988–1994 to 49.9% during 2007–2010. Similar race/ethnicity differences were observed for males. These data, along with data from NHANES survey years 1976–1980,30 indicate that non-Hispanic Blacks had higher overall seroprevalence than non-Hispanic Whites in each survey period.
NHANES data also show that among adolescents aged 14–19 years, HSV-1 seroprevalence has significantly decreased by almost 23%, from 39.0% during 1999–2004 to 30.1% during 2005–2010, indicating declining orolabial infection in this age group.28 HSV-2 seroprevalence in this age group was much lower, less than 2% in both time periods.28 Other studies have found that genital HSV-1 infections are increasing among young adults.31,32 This has been attributed, in part, to the decline in orolabial HSV-1 infections, because those who lack HSV-1 antibodies at sexual debut are more susceptible to genital HSV-1 infection;28,33 increasingly common oral sex behavior among adolescents and young adults also has been suggested as a contributing factor.28,34 The absence of HSV-1 antibodies also increases the likelihood of developing symptomatic disease from newly acquired (i.e., primary) genital HSV-2 infection.35 Young women may therefore be increasingly likely to first acquire HSV-1 infection genitally, or acquire a primary genital HSV-2 infection during their child-bearing years,33,36 and first-episode primary HSV infection during pregnancy increases the risk of neonatal HSV transmission.33,37
For information on neonatal HSV infections, see Special Focus Profiles, STDs in Women and Infants.
Trichomonas Vaginalis
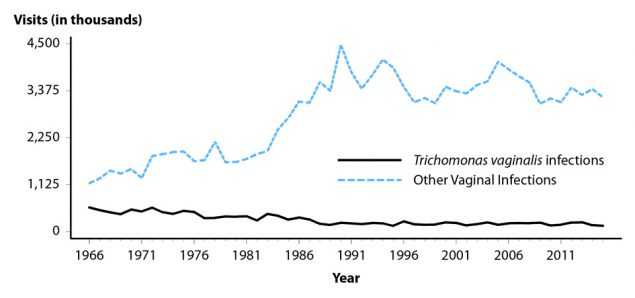
Figure 50. Trichomonas vaginalis and Other Vaginal Infections Among Women — Initial Visits to Physicians’ Offices, United States, 1966–2015
Trichomonas vaginalis is a common sexually transmitted protozoal infection associated with adverse health outcomes such as preterm birth and symptomatic vaginitis.4,38,39 It is not a nationally reportable condition, and trend data are limited to estimates of initial physician office visits for this condition from the National Disease and Therapeutic Index (NDTI; see Section A2.5 in the Appendix) (Figure 50, Table 44). Visits appear to be fairly stable since the 1990’s; the number of initial visits for Trichomonas vaginalis infection in 2015 was 139,000. The 2016 NDTI data were not obtained in time to include them in this report. NHANES data from 2001–2004 indicated an overall Trichomonas vaginalis infection prevalence of 3.1%, with the highest prevalence of 13.3% observed among non-Hispanic Blacks.39
References
1. Lewis DA. Chancroid: Clinical Manifestations, Diagnosis, and Management. Sex Transm Infect 2003; 79:68–71.
2. Schulte JM, Martich FA, Schmid GP. Chancroid in the United States, 1981–1990: Evidence for Underreporting of Cases. MMWR Morb Mortal Wkly Rep Surveill Summ 1992; 41(SS-3):57–61.
3. Mertz KJ, Trees D, Levine WC, et al. Etiology of Genital Ulcers and Prevalence of Human Immunodeficiency Virus Coinfection in 10 US Cities. J Infect Dis 1998; 178(6):1795–1798.
4. Satterwhite CL, Torrone E, Meites E, et al. Sexually Transmitted Infections among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex Transm Dis 2013; 40(3):187–193.
5. de Villiers E-M, Fauquet C, Broker TR, et al. Classification of Papillomaviruses. Virology 2004; 324:17–27.
6. Insinga RP, Perez G, Wheeler CM, et al. Incident Cervical HPV Infections in Young Women: Transition Probabilities for CIN and Infection Clearance. Cancer Epidemiol Biomarkers Prev 2011; 20(2):287–296.
7. McQuillan G, Kruszon-Moran D, Markowitz LE, et al. Prevalence of HPV in Adults Aged 18–69: United States, 2011–2014. NCHS Data Brief, No 280. Hyattsville, MD: National Center for Health Statistics; 2017.
8. Saraiya M, Unger ER, Thompson TD, et al. US Assessment of HPV types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J Natl Cancer Inst 2015; 107(6):djv086.
9. Clifford GM, Rana RK, Franceschi S, et al. Human Papillomavirus Genotype Distribution in Low-Grade Cervical Lesions: Comparison by Geographic Region and with Cervical Cancer. Cancer Epidemiol Biomarkers Prev 2005; 14(5):1157–1164.
10. Porras C, Rodriguez AC, Hildesheim A, et al. Human Papillomavirus Types by Age in Cervical Cancer Precursors: Predominance of Human Papillomavirus 16 in Young Women. Cancer Epidemiol Biomarkers Prev 2009; 18(3):863–865.
11. Garland SM, Steben M, Sings HL, et al. Natural History of Genital Warts: Analysis of the Placebo Arm of 2 Randomized Phase III Trials of a Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Vaccine. J Infect Dis 2009; 199(6):805–814.
12. Gissmann L, Wolnik L, Ikenberg H, et al. Human Papillomavirus Types 6 and 11 DNA Sequences in Genital and Laryngeal Papillomas and in Some Cervical Cancers. Proc Natl Acad Sci USA 1983; 80(2): 560–563.
13. Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep Recomm Rep 2007;
14. Centers for Disease Control and Prevention. FDA Licensure of Quadrivalent Human Papillomavirus Vaccine (HPV4, Gardasil) for Use in Males and Guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59(20):630–632.
15. Centers for Disease Control and Prevention. FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59(20):626–629.
16. Stokley S, Jeyarajah J, Yankey D, et al. Human Papillomavirus Vaccination Coverage Among Adolescents, 2007–2013, and Postlicensure Vaccine Safety Monitoring, 2006-2014 — United States. MMWR Morb Mortal Wkly Rep 2014; 63(29):620–624.
17. Petrosky E, Bocchini Jr. JA, Hariri S, et al. Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2015; 64(11):300–304.
18. Centers for Disease Control and Prevention. Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males — Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60(50):1705–1708.
19. HealthyPeople.gov. Healthy People 2020 Topics & Objectives. Immunization and Infectious Diseases. Objectives IID-11.4 and IID-11.5. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed July 20, 2016.
20. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage among Adolescents Aged 13–17 years — United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858.
21. Markowitz LE, Gui L, Hariri S, et al. Prevalence of HPV After Introduction of the Vaccination Program in the United States. Pediatrics 2016; 137(3):e20151968.
22. Gargano JW, Unger ER, Liu G, et al. Prevalence of Genital Human Papillomavirus in Males, United States, 2013–2014. J Infect Dis 2017; 215(7):1070–1079.
23. Flagg EW, Torrone EA, Weinstock H. Ecological Association of Human Papillomavirus Vaccination with Cervical Dysplasia Prevalence in the United States, 2007–2014. Am J Public Health 2016; 106(12):2211–2218.
24. Flagg EW, Schwartz R, Weinstock H. Prevalence of Anogenital Warts Among Participants in Private Health Plans in the United States, 2003–2010: Potential Impact of Human Papillomavirus Vaccination. Am J Public Health 2013; 103(8):1428–1435.
25. Smith JS, Robinson NJ. Age-Specific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. J Infect Dis 2002; 186(Suppl 1):S3–S28.
26. Corey L, Wald A. Genital Herpes. In: Holmes KK, Sparling FP, Stamm WE, et al., eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 2008:399–437.
27. Kimberlin DW, Rouse DJ. Genital Herpes. N Engl J Med 2004; 350(19):1970–1977.
28. Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of Herpes Simplex Virus Types 1 and 2 — United States, 1999–2010. J Infect Dis 2014; 209(3):325–333.
29. Fanfair RN, Zaidi A, Taylor LD, et al. Trends in Seroprevalence of Herpes Simplex Virus Type 2 among Non-Hispanic Blacks and Non-Hispanic Whites Aged 14 to 49 Years — United States, 1988 to 2010. Sex Transm Dis 2013; 40(11):860–864.
30. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in Herpes Simplex Virus Type 1 and Type 2 Seroprevalence in the United States. JAMA 2006; 296(8):964–973.
31. Bernstein DI, Bellamy AR, Hook EW III, et. al. Epidemiology, Clinical Presentation, and Antibody Response to Primary Infection with Herpes Simplex Virus Type 1 and Type 2 in Young Women. Clin Infect Dis 2013; 56:344–351.
32. Roberts CM, Pfister JR, Spear SJ. Increasing Proportion of Herpes Simplex Virus Type 1 as a Cause of Genital Herpes Infection in College Students. Sex Transm Dis 2003; 30(10):797–800.
33. Kimberlin DW. The Scarlet H. J Infect Dis 2014; 209(3):315–317.
34. Copen CE, Chandra A, Martinez G. Prevalence and Timing of Oral Sex with Opposite-sex Partners among Females and Males Aged 15–24 Years: United States, 2007–2010. National Health Statistics Reports; No. 56. Hyattsville, MD: National Center for Health Statistics; 2012.
35. Langenberg AGM, Corey L, Ashley RL, et al. A Prospective Study of New Infections with Herpes Simplex Virus Type 1 and Type 2. N Engl J Med 1999; 341:1432–1438.
36. Sampath A, Maduro G, Schillinger JA. Infant Deaths Due to Herpes Simplex Virus, Congenital Syphilis, and HIV in New York City. Pediatrics 2016; 137(4):e20152387.
37. Brown ZA, Wald A, Morrow RA, et al. Effect of Serologic Status and Cesarean Delivery on Transmission Rates of Herpes Simplex Virus from Mother to Infant. JAMA 2003; 289(2):203–209.
38. French JI, McGregor JA, Parker R. Readily Treatable Reproductive Tract Infections and Preterm Birth among Black Women. Am J Obstet Gynecol 2006; 194:1717–1727.
39. Sutton M, Sternberg M, Koumans EH, et al. The Prevalence of Trichomonas vaginalis Infection Among Reproductive-Age Women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326.
- Page last reviewed: September 26, 2017
- Page last updated: September 26, 2017
- Content source:


 ShareCompartir
ShareCompartir