Elimination of Mother-to-Child HIV Transmission (EMCT) in the United States

In collaboration with national experts and federal partners, the Centers for Disease Control and Prevention (CDC) has developed a new framework directed at the goal of eliminating mother-to-child (perinatal) HIV transmission (EMCT) in the United States. The framework is illustrated in Figure 1 [the numbers in superscript below refer to the corresponding numbered activities in the figure] and differs from the previous approach to PMTCT in its consistent use of public health personnel to 1) assure that HIV care includes comprehensive reproductive health, family planning services and preconception care and that women of childbearing age are HIV tested according to CDC recommendations1; and 2) conduct comprehensive, real-time case finding2 of all HIV-infected pregnant women and their exposed infants. These two large tasks are conducted under the concept of Perinatal HIV Services Coordination (PHSC). PHSC in each state (or jurisdiction) could assure comprehensive, real-time case finding. It may be helpful to have these functions combined with other related activities, such as the prevention of other perinatally acquired infections. With the financial realities facing all levels of government, and the fact that most HIV-infected pregnant women are, in fact, in care, it will not be possible—and may not be necessary—for PHSC to actively monitor each woman, once it has been determined that the woman is in care. On the other hand, for those women recognized as not in care, finding cases in real time would enable the conduct and coordination of the following components of the EMCT framework:
- Comprehensive Care (facilitation of comprehensive clinical and psychosocial HIV services for women and infants3; )
- Case Review and Community Action (FIMR-HIV) to identify and address missed prevention opportunities and local systems issues through continuous quality improvement4;
- Research and long-term monitoring to develop and ensure safe and efficacious PMCT interventions5; and Data reporting for HIV surveillance and EMCT evaluation6.
Reproductive Health, Family Planning Services and Preconception Care
Increased identification of HIV infection prior to pregnancy, and maintenance of more women in care would result in more opportunities to optimize HIV-infected women’s health prior to and between pregnancies, particularly with new guidelines recommending early antiretroviral (ARV) treatment. Proper attention to women’s HIV care inclusive of reproductive health and family planning will support women prevent unintended pregnancy.
Comprehensive real-time case finding
A “case” is a pregnancy in an HIV-infected woman. Case-finding requires both universal HIV testing of pregnant women and a mechanism for detecting pregnancy among women with diagnosed HIV infection and is distinct from traditional HIV surveillance because cases must be identified in real time.
Comprehensive Care
Clinical management for HIV-infected pregnant women and their infants is credited with much of the documented reduction in MCT. Local expertise in clinical care has been nurtured by the support for clinical activities provided by the Health Resources and Services Administration (HRSA), under the Ryan White HIV/AIDS Program. Assuring linkages to these clinical providers is a key function of the PHSC. Under the financial restraints of recent years, jurisdictions will need to determine the degree of their involvement with individual HIV-infected pregnant women. Recent national health-care reform may enhance EMCT through reimbursement for preventive care and by facilitating access to care.
Because MCT is frequently the result of failures of local health systems, CDC has worked with partners to develop a community-based, continuous quality improvement methodology. This methodology is modeled after the HRSA-funded Fetal-Infant Mortality Review (FIMR) program. CDC funds the FIMR-HIV Prevention Methodology (FHPM) National Resource Center to support scale-up of this methodology in new sites such as health departments funded through CDC’s HIV Prevention cooperative agreements. With consent, FHPM summarizes the experience from the mother’s perspective through an interview and medical records abstraction. This de-identified information is presented to a multidisciplinary case review team to assess missed opportunities related to MCT and to develop recommendations for local system improvements. These recommendations are presented to community action teams of locally recognized persons who are in a position to implement the suggested system improvements. Cases of MCT occur in diverse and ever-changing environments, so utilizing a proven methodology to assess and improve local systems is particularly important. Such systems improvements could also affect other perinatal infections (e.g., hepatitis B infection and syphilis) and maternal and child health services overall.
Research and Long-term Monitoring
The prevention regimens used are evolving and expose thousands of children to antiretroviral medications every year. Research to monitor the long-term safety and efficacy of interventions for both women and ARV-exposed children needs a long-term plan and sustained action.
Data Reporting
Accurate data reporting is necessary to direct the activities described above, and should include surveillance of all infants born to HIV-infected women (“perinatal HIV exposure”). Without exposure reporting, it is challenging if not impossible to understand progress or lack thereof toward the goal of EMCT.
This framework describes how health care and public health systems may interact to eliminate mother-to-child HIV transmission in the United States. Considering the multifaceted approach of the framework, as well as the current strain on resources, reaching elimination will require coordination and active collaboration across multiple agencies at the national, state and local level. However, preventing perinatal transmission at the individual level includes a series of clinical interventions. These interventions are outlined in the Perinatal HIV Prevention Cascade (Figure 2) adapted from the Institute of Medicine 1998 report, Reducing the odds: Preventing perinatal transmission of HIV in the United States.
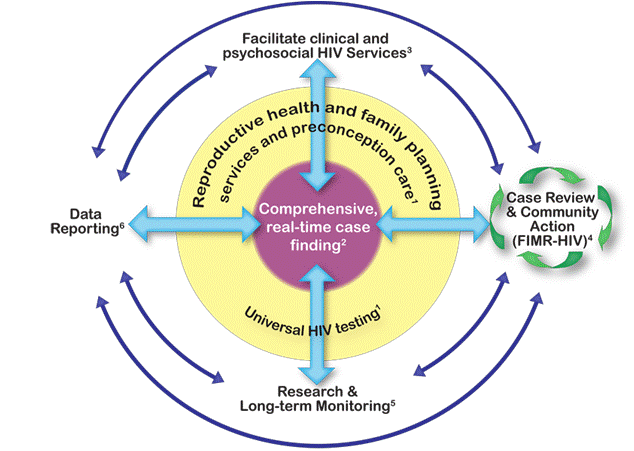
Figure 1 – Framework to Eliminate Mother-to-Child HIV Transmission (EMCT)
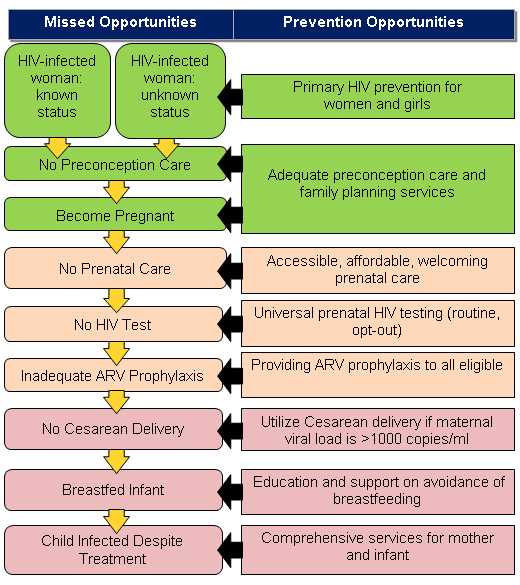
Figure 2 – Perinatal HIV Prevention Cascade
Source Report: Institute of Medicine, 1998
1 Reproductive health and family planning services, preconception care, and universal HIV testing are essential components of EMCT and facilitate 2 comprehensive real-time case finding of all HIV-infected pregnant women. Real-time case finding enables: 3 comprehensive clinical care and social services for women and infants; 4 detailed review of select cases to identify and address missed prevention opportunities and local systems issues through continuous quality improvement; 5 research and long-term follow-up to develop and ensure safe, efficacious interventions for EMCT; 6 thorough data reporting for HIV surveillance and EMCT evaluation.
- Page last reviewed: August 28, 2017
- Page last updated: August 28, 2017
- Content source: Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention


 ShareCompartir
ShareCompartir