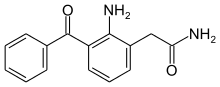
Nepafenac
Nepafenac (brand name Nevanac or Ilevro) is a nonsteroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop 0.1% solution (Nevanac) or 0.3% solution (Ilevro). It is used to treat pain and inflammation associated with cataract surgery.[1] Nepafenac is a prodrug of amfenac, an inhibitor of COX-1 and COX-2 activity.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Amnac, Ilevro |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606007 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Ophthalmic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.414 |
| Chemical and physical data | |
| Formula | C15H14N2O2 |
| Molar mass | 254.28 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
Nepafenac is indicated for use in the treatment of pain and inflammation following cataract surgery.[1][4][5] The usual dose is one drop, thrice a day, in each affected eye beginning one day prior to cataract surgery, continued on the day of surgery and through the first two weeks of the postoperative period.[1]
Pharmacology
Adverse events
Side effects include headache; runny nose; pain or pressure in the face; nausea; vomiting; and dry, itchy, sticky eyes.[6] Serious side effects include red or bloody eyes; foreign body sensation in the eye; sensitivity to light; decreased visual acuity; seeing specks or spots; teary eyes; or eye discharge or crusting.[6]
Regulatory
Nevanac
On February 25, 2005, Alcon filed an NDA with the FDA for Nevanac 0.1%.[7] Results from the two trials referenced in the NDA (Phase 2/3 study C-02-53; Phase 3 study C-03-32) have not been published.[8] Study C-02-53 consisted of 228 patients across 10 centers in the United States.[9] Study C-03-32 consisted of 522 patients across 22 centers in the United States. [9] The efficacy results presented were confirmed in a study published in 2007.[10]
Nevanac was approved by the FDA on August 19, 2005 with application number 021-862.[11]
Ilevro
An NDA for Ilevro was filed on December 15, 2011.[12] In a one-month study, no new toxicities arose in the new formulation of nepafenac.[13] Safety and efficacy information was derived from the previous Nevanac application.[13] In June 2010, a confirmatory study began (Study C09055) consisting of over 2000 patients from 49 US sites and 37 European sites.[14][15] A second phase 3 trial (Study C11003) was conducted in a population of 1,342 patients at 37 sites across the United States which failed to demonstrate superiority over Nevanac in an altered dosing regimen.[14]
Ilevro was approved by the FDA on October 16, 2012 with application number 203-491.[16]
Commercialization
Both Nevanac and Ilevro are manufactured and sold by Alcon, Inc. [4][5]. Alcon is currently a division of Novartis International AG, which is primarily based out of Switzerland. [17] Alcon, Inc. also holds locations in both Switzerland and the United States.[18] The company has gone through several name changes, from Alcon Laboratories, Inc. to Alcon Universal, Ltd., to Alcon, Inc. [18]
Nevanac entered the market in 2005 as a product of Alcon, at the time a subsidiary of Nestlé.[19] On April 6, 2008, Novartis agreed to purchase approximately 74 million shares of Alcon from Nestlé at $143.18 per share.[19] On January 4, 2010, Novartis agreed to purchase all remaining shares of Alcon from Nestlé, totalling 156 million shares or 77% of the shares in the company.[19] At the time of the purchase, a proposal for a merger under Swiss merger law was given to the Alcon board of directors.[19] The merger was agreed upon on December 15, 2010, making Alcon "the second largest division within Novartis."[19] The merger was completed on April 8, 2011.[20]
Ilevro was launched by Alcon on January 21, 2013.[21] In 2014 and 2015, net sales by Alcon grew, contributed to in part by the increased volume in sales of Ilevro.[22][23][24] That financial year, Novartis reported $18 billion in total financial debt. [22] That figure has grown steadily since. In 2016, Novartis reported a total debt of $23.8 billion[25], up from the $21.9 billion reported in 2015 [24] and the $20.4 billion reported in 2014. [23] As of May 2017, Novartis is estimated to be worth $193.2 billion.[26]
On January 27, 2016, Alcon was moved to become a branch of the Innovative Medicines Division at Novartis.[25] Early in 2016, Alcon formed agreements with both TrueVision and PowerVision, and acquired Transcend Medical. [25] As of January 2017, Novartis is weighing options for Alcon in the business structure.[25]
Cost
As of 2015, roughly 266,751 Medicare Part D patients have been prescribed Ilevro as part of their therapeutic regimen for cataract surgery, earning Novartis approximately $95.2 million in 2015 from the 411,000 claims filed for the drug.[27] The average yearly cost for a Medicare Part D patient for Ilevro is $94.[27] The estimated per unit price in the United States for Nevanac is $88.93.[28] For Ilevro, the per unit price for the 1.7 mL bottle averages $156.89 and the per unit price for the 3 mL bottle averages $88.91.[29] Average price for both drugs is similar.[28][29]
Generic formulations are not yet available for either drug.[28][29]
Commercial risks
Alcon faced declining growth in 2016, having faced challenges in development and marketing of new products. [25]
Marketing
Novartis maintains a detailing unit geared toward health professionals consisting of over 3,000 employees within the United States and an additional 21,000 worldwide.[25] Novartis is also seeking to expand direct-to-consumer advertising and entrance into speciality product markets. [25] Novartis also notes the influence of position and preference on US Centers for Medicare & Medicaid formularies in expanding their market value. [25]
Nepafenac, Nevanac, and Ilevro are all absent from the 2016 Annual Report issued from Novartis.[25] Specific marketing aims for these drugs cannot be ascertained.
Intellectual property
There are currently seven U.S. patents filed that are directly associated with the modernized formulations of nepafenac, all stemming from Novartis.[30]There are three patents associated with Nevanac that are still active[31] and four associated with Ilevro.[32]The earliest patent related to the modern formulations of nepafenac was approved on June 11, 2002 after being filed in 1999 by Bahram Asgharian.[33] A patent was filed by Warren Wong, associated with Alcon, Inc. based out of Fort Worth, Texas, on December 2, 2005 for aqueous suspensions of nepafenac.[34] Another patent for a nepafenac-based drug was filed on May 8, 2006 by Geoffrey Owen, Amy Brooks, and Gustav Graff.[35] A patent was filed by Masood A. Chowhan and Huagang Chen on February 9, 2007 and approved on May 24, 2011[36], followed closely by a patent filed by Warren Wong on September 23, 2010 and approved on December 6, 2011.[37] Masood A. Chowhan, Malay Ghosh, Bahram Asgharian, and Wesley Wehsin Han filed another patent on December 1, 2010 and approved on December 30, 2014.[38] The most recent patent was filed by Masood A. Chowhan, Malay Ghosh, Bahram Asgharian, and Wesley Weshin Han on November 12, 2014 and approved on May 30, 2017.[39] These patents are in effect until dates ranging between July 17, 2018 and March 31, 2032.[32]
Novartis also maintains patents on nepafenac in 26 countries outside the United States.[40]
References
- Nepafenac Monograph
- Drugbank: Nepafenac
- Lira, R. P.; Fulco, E. A.; Chaves, A.; Da Costa Pinto, F.; Arieta, F. R.; Lira, C. E. (2012). "Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: A randomized trial". Indian Journal of Ophthalmology. 60 (4): 277–281. doi:10.4103/0301-4738.98705. PMC 3442462. PMID 22824596.
- "Nevanac (nepafenac) ophthalmic suspension label" (PDF). FDA. FDA. Retrieved 25 October 2017.
- "Ilevro Full Prescribing Information" (PDF). Novartis. Novartis. Retrieved 25 October 2017.
- "Nepafenac Ophthalmic". MedlinePlus. U.S. National Library of Medicine. Retrieved October 31, 2017.
- "Nevanac Approval Package" (PDF). FDA. FDA. Retrieved October 31, 2017.
- Gaynes, BI; Onyekwuluje, A (June 2008). "Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension". Clinical Ophthalmology (Auckland, N.Z.). 2 (2): 355–68. doi:10.2147/opth.s1067. PMC 2693998. PMID 19668727.
- "Nevanac Statistical Review" (PDF). FDA. FDA. Retrieved October 31, 2017.
- Lane, SS; Modi, SS; Lehmann, RP; Holland, EJ (January 2007). "Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery". Journal of Cataract and Refractive Surgery. 33 (1): 53–8. doi:10.1016/j.jcrs.2006.08.043. PMID 17189793.
- "Drug Approval Package: Nevanac (Nepafenac) NDA #021862". FDA. FDA. Retrieved October 27, 2017.
- "Ilevro Approval Package" (PDF). FDA. FDA. Retrieved October 31, 2017.
- "203491 Pharmacology Review" (PDF). US FDA. FDA. Retrieved October 31, 2017.
- "Ilevro Statistical Review" (PDF). FDA. FDA. Retrieved October 31, 2017.
- "Confirmatory Study Nepafenac 0.3%". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved October 31, 2017.
- "Drug Approval Package: Nepafenac NDA #203491". FDA. FDA. Retrieved October 27, 2017.
- "About Us". Novartis. Novartis. Retrieved October 27, 2017.
- "Nevanac Administrative Documents and Correspondence" (PDF). FDA. FDA. Retrieved October 27, 2017.
- "Alcon Annual Report 2010" (PDF). SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 30, 2017.
- "Alcon Form 15". SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 30, 2017.
- "Alcon Launches ILEVRO™ (nepafenac ophthalmic suspension) 0.3%, a New Non-Steroidal Anti-Inflammatory Drug, for the Treatment of Pain and Inflammation Associated with Cataract Surgery". Alcon. Alcon Global. Retrieved October 30, 2017.
- "Form 20-F". SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 30, 2017.
- "Form 20-F". SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 30, 2017.
- "Form 20F". SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 30, 2017.
- "Form 20-F". SEC EDGAR. United States Securities and Exchange Commission. Retrieved October 31, 2017.
- "Novartis on the Forbes Top Multinational Performers List". Forbes.com. Forbes Media LLC. Retrieved October 31, 2017.
- "Prescriber Checkup: Ilevro". Prescriber Checkup. ProPublica. Retrieved October 30, 2017.
- "Nevanac Prices, Coupons and Patient Assistance Programs". Drugs.com. Retrieved October 31, 2017.
- "Ilevro Prices, Coupons and Patient Assistance Programs". Drugs.com. Retrieved October 31, 2017.
- "Nepafenac". U.S. Patents. PharmaCompass. Retrieved October 30, 2017.
- "Generic Nevanac Availability". Drugs.com. Retrieved October 31, 2017.
- "Generic Ilevro Availability". Drugs.com. Retrieved October 31, 2017.
- "United States Patent Application: 6403609". United States Patent and Trademark Office. United States PTO. Retrieved October 31, 2017.
- "United States Patent Application: 0060122277". United States Patent and Trademark Office. United States PTO. Retrieved October 30, 2017.
- "United States Patent Application: 0060257487". United States Patent and Trademark Office. United States PTO. Retrieved October 27, 2017.
- "United States Patent: 7947295". United States Patent and Trademark Office. United States PTO. Retrieved October 31, 2017.
- "United States Patent: 8071648". United States Patent and Trademark Office. United States PTO. Retrieved October 31, 2017.
- "United States Patent: 8921337". United States Patent and Trademark Office. United States PTO. Retrieved October 31, 2017.
- "United States Patent: 9662398". United States Patent and Trademark Office. United States PTO. Retrieved October 31, 2017.
- "Nepafenac - Generic Drug Details". DrugPatentWatch. thinkBiotech LLC. Retrieved October 31, 2017.
- "Complaint". RPX Insight. RPX Corporation. Retrieved October 31, 2017.
- "Amended Complaint". RPX Insight. RPX Corporation. Retrieved October 31, 2017.