Ceftolozane/tazobactam
Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a combination antibiotic. It is indicated for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults.[1] Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics.[2] It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.
 | |
| Clinical data | |
|---|---|
| Trade names | Zerbaxa |
| License data | |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
| Formula | C23H30N12O8S2 |
| Molar mass | 666.689 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ceftolozane is combined with the β-lactamase inhibitor tazobactam, which protects ceftolozane from degradation.[3] It was approved for medical use in the United States in 2014.
Chemical structure
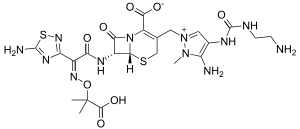
Ceftolozane contains a 7-aminothiadiazole, affording increased activity against gram-negative organisms, as well as an alkoximino group, providing stability against many β-lactamases. Ceftolozane has a dimethylacetic acid moiety that contributes to enhanced activity against Pseudomonas aeruginosa. The addition of a bulky side chain (a pyrazole ring) at the 3-position prevents hydrolysis of the β-lactam ring via steric hindrance.[4]
Tazobactam is a penicillinate sulfone β-lactamase inhibitor, which prevents hydrolysis of the amide bond of the β-lactam molecules by β-lactamase enzymes.[5]
Mechanism of action
Ceftolozane exerts bactericidal activities against susceptible gram-negative and gram-positive infections by inhibiting essential penicillin-binding proteins (PBPs), which are required for peptidoglycan cross-linking for bacterial cell wall synthesis, resulting in inhibition of cell wall synthesis and subsequent cell death. Ceftolozane is an inhibitor of PBPs of Pseudomonas aeruginosa (e.g. PBP1b, PBP1c, and PBP3) and E. coli (e.g., PBP3).[6][7]
Tazobactam is a potent β-lactamase inhibitor of most common class A and C β-lactamases. Tazobactam has little clinically relevant in vitro activity against bacteria due to its reduced affinity to penicillin-binding proteins; however, it is an irreversible inhibitor of some β-lactamases (certain penicillinases and cephalosporinases) and can covalently bind to some chromosomal and plasmid-mediated bacterial beta-lactamases.[6]
The addition of tazobactam strengthens the therapeutic response to ceftolozane, giving it the ability to treat a broader range of bacterial infections and resistant organisms.[8]
Pharmacokinetics
Absorption and distribution
Ceftolozane–tazobactam is available as a 2:1 fixed combination (such that a 1.5-g dose of ceftolozane–tazobactam is composed of 1 g of ceftolozane and 500 mg of tazobactam)[9]. Ceftolozane-tazobactam is administered intravenously. For both ceftolozane and tazobactam, the peak plasma concentration occurs immediately after a 60-minute infusion, with a time to maximum concentration of approximately one hour. The binding of ceftolozane to human plasma proteins is approximately 16% to 21%, while the binding of tazobactam is approximately 30%. The mean steady-state volume of distribution in healthy adult males after a single 1.5 g IV dose is 13.5 L for ceftolozane and 18.2 L for tazobactam, which is similar to extracellular fluid volume. Tissue distribution of ceftalozone-tazobactam is rapid and shows good penetration into the lung, rendering it an ideal treatment for bacterial pneumonia.[8]
Metabolism and elimination
The metabolism and excretion of ceftolozane are similar to those of most β-lactam antimicrobial agents. Certolozane is not metabolized to any significant extent and thus predominantly eliminated unchanged in the urine.[10][11] Tazobactam is partially metabolized to an inactive metabolite, and both drug and metabolite are excreted in the urine (80% as unchanged drug).[12]
The half-life of ceftolozane is 2.5–3.0 hours, and the half-life of tazobactam is approximately 1.0 hour; the clearance of both drugs is directly proportional to renal function. Tazobactam primarily undergoes renal excretion via active tubular secretion. Coadministration of ceftolozane with tazobactam does not result in an interaction, since ceftolozane is primarily eliminated by glomerular filtration.[13][12]
Spectrum of activity
The in vitro activity of ceftolozane–tazobactam has been examined in five surveillance studies of isolates from Europe and North America.[14] In these studies, ceftolozane–tazobactam was notable for its activity against Pseudomonas aeruginosa, a moderately common cause of hospital-acquired infections that is commonly multi-drug resistant. Ninety percent of Pseudomonas aeruginosa isolates were inhibited by a ceftolozane–tazobactam at a concentration of 4 μg/mL (MIC90), making it the most potent anti-pseudomonal antibiotic in clinical use.
In these same studies, ceftolozane–tazobactam exhibited MIC90 values of <1 μg/mL for Escherichia coli, Citrobacter koseri, Morganella morganii, Proteus mirabilis, Salmonella species, and Serratia marcescens. Somewhat poorer activity is observed for the Klebsiella and Enterobacter species, with the MIC90 for extended spectrum beta-lactamase expressing Klebsiella pneumoniae being >32 μg/mL.
Adverse drug reactions
The adverse-event profile of ceftolozane/tazobactam from two phase 2 trials (comparing either ceftolozane alone or in combination with tazobactam to ceftazidime or meropenem) suggests that ceftolozane/tazobactam is well tolerated. The most common AEs reported with ceftolozane/tazobactam were headache (5.8%), constipation (3.9%), hypertension (3%), nausea (2.8%), and diarrhea (1.9%).[15]
Drug interactions
Based on previous trial data and ongoing clinical trials, no significant drug–drug or food–drug interactions have been associated with ceftolozane/tazobactam administration. However, drug–drug interactions similar to those observed with the cephalosporin class of antimicrobials and β-lactamase inhibitors should be considered as potential interactions until further drug–drug interactions have been completely elucidated. Moreover,as a result of drug accumulation in renal impairment, caution should be taken when coadministering ceftolozane/tazobactam with other renally eliminated medications due to possible nephrotoxicity[15]
Chemical synthesis
Researchers at Cubist Pharmaceuticals (prior to the acquisition of Cubist by Merck) discovered and developed a synthesis of ceftolozane sulfate based on a palladium-mediated coupling in the presence of the cephalosporin nucleus, marking a significant advancement in the chemistry of cephalosporin antibiotics. This chemistry was determined to be general to the family of cephalosporin antibiotics. Key elements of the coupling reaction were the use of a designed, electron-deficient phosphite ligand in tandem with the addition of an exogenous chloride scavenging reagent, which functioned through the in situ precipitation of potassium chloride. This work is described only in the patent literature.[16]
References
- "Archived copy". Archived from the original on 2015-05-09. Retrieved 2015-06-02.CS1 maint: archived copy as title (link)
- Long TE, Williams JT (October 2014). "Cephalosporins currently in early clinical trials for the treatment of bacterial infections". Expert Opinion on Investigational Drugs. 23 (10): 1375–87. doi:10.1517/13543784.2014.930127. PMID 24956017.
- Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, et al. (January 2014). "Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli". Drugs. 74 (1): 31–51. doi:10.1007/s40265-013-0168-2. PMID 24352909.
- Murano K, Yamanaka T, Toda A, Ohki H, Okuda S, Kawabata K, et al. (March 2008). "Structural requirements for the stability of novel cephalosporins to AmpC beta-lactamase based on 3D-structure". Bioorganic & Medicinal Chemistry. 16 (5): 2261–75. doi:10.1016/j.bmc.2007.11.074. PMID 18082409.
- Drawz SM, Bonomo RA (January 2010). "Three decades of beta-lactamase inhibitors". Clinical Microbiology Reviews. 23 (1): 160–201. doi:10.1128/CMR.00037-09. PMC 2806661. PMID 20065329.
- Shortridge D, Pfaller MA, Castanheira M, Flamm RK (June 2018). "Antimicrobial Activity of Ceftolozane-Tazobactam Tested Against Enterobacteriaceae and Pseudomonas aeruginosa with Various Resistance Patterns Isolated in U.S. Hospitals (2013-2016) as Part of the Surveillance Program: Program to Assess Ceftolozane-Tazobactam Susceptibility". Microbial Drug Resistance. 24 (5): 563–577. doi:10.1089/mdr.2017.0266. PMID 29039729.
- Snydman DR, McDermott LA, Jacobus NV (February 2014). "Activity of ceftolozane-tazobactam against a broad spectrum of recent clinical anaerobic isolates". Antimicrobial Agents and Chemotherapy. 58 (2): 1218–23. doi:10.1128/AAC.02253-13. PMC 3910869. PMID 24277025.
- Hong MC, Hsu DI, Bounthavong M (November 2013). "Ceftolozane/tazobactam: a novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination". Infection and Drug Resistance. 6: 215–23. doi:10.2147/idr.s36140. PMC 3848746. PMID 24348053. Retrieved 2019-06-23.
- Cluck D, Lewis P, Stayer B, Spivey J, Moorman J (December 2015). "Ceftolozane-tazobactam: A new-generation cephalosporin". American Journal of Health-System Pharmacy. 72 (24): 2135–46. doi:10.2146/ajhp150049. PMID 26637512.
- Chandorkar G, Xiao A, Mouksassi MS, Hershberger E, Krishna G (February 2015). "Population pharmacokinetics of ceftolozane/tazobactam in healthy volunteers, subjects with varying degrees of renal function and patients with bacterial infections". Journal of Clinical Pharmacology. 55 (2): 230–9. doi:10.1002/jcph.395. PMC 4303958. PMID 25196976.
- Wooley M, Miller B, Krishna G, Hershberger E, Chandorkar G (2014-04-01). "Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam". Antimicrobial Agents and Chemotherapy. 58 (4): 2249–55. doi:10.1128/AAC.02151-13. PMID 24492369.
- Goodlet KJ, Nicolau DP, Nailor MD (2016-12-01). "Ceftolozane/tazobactam and ceftazidime/avibactam for the treatment of complicated intra-abdominal infections". Therapeutics and Clinical Risk Management. 12: 1811–1826. doi:10.2147/tcrm.s120811. PMID 27942218. Retrieved 2019-06-23.
- Miller B, Hershberger E, Benziger D, Trinh M, Friedland I (June 2012). "Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses". Antimicrobial Agents and Chemotherapy. 56 (6): 3086–91. doi:10.1128/AAC.06349-11. PMID 22450972.
- "Medical review" (PDF). fda.gov.
- Sorbera M, Chung E, Ho CW, Marzella N (December 2014). "Ceftolozane/Tazobactam: a new option in the treatment of complicated gram-negative infections". Pharmacy & Therapeutics. 39 (12): 825–32. PMC 4264669. PMID 25516692.
- U. S. Patent Application No. WO2016025839 (A1) 2016