Cefuroxime axetil
Cefuroxime axetil, sold under the brand name Zinnat among others, is a second generation oral cephalosporin antibiotic.
 | |
| Clinical data | |
|---|---|
| Trade names | Zinnat, Ceftin, Ceftum |
| Other names | Cefuroxime 1-acetoxyethyl ester |
| Routes of administration | Oral, IV, IM |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Metabolism | Cefuroxime is not metabolized, Axetil is metabolized to acetaldehyde and acetic acid. |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.166.374 |
| Chemical and physical data | |
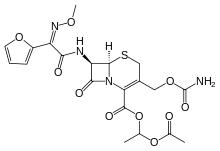
| Formula | C20H22N4O10S |
| Molar mass | 510.475 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It is an acetoxyethyl ester prodrug of cefuroxime which is effective orally.[1] The activity depends on in vivo hydrolysis and release of cefuroxime tablets.
It was patented in 1976 and approved for medical use in 1987.[2]
History
It was discovered by Glaxo now GlaxoSmithKline and introduced in 1987.[3] It was approved by FDA on December 28, 1987.[4] It is available by GSK as Ceftin in US[5] and Ceftum in India.[6]
See also
References
- Walter Sneader (2005-06-23). Drug Discovery: A History. John Wiley, Chichester, UK. ISBN 0-471-89979-8.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 494. ISBN 9783527607495.
- "Our history - About GSK". GlaxoSmithKline. Archived from the original on 2011-05-14.
- "Cefuroxime Axetil Monograph for Professionals". Drugs.com. Retrieved 2018-04-22.
- "Brands". Gsksource.com. 2018-03-22. Retrieved 2018-04-22.
- "Our products". GlaxoSmithKline.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.