Abacavir/lamivudine/zidovudine
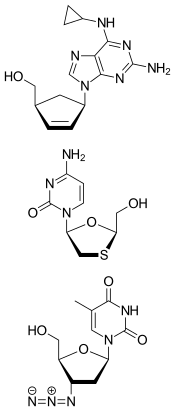
Abacavir/lamivudine/zidovudine, sold under the trade name Trizivir, is a medication for HIV infection.[1] It is a fixed dose combination of three reverse transcriptase inhibitors patented by GlaxoSmithKline and marketed by a joint venture with Pfizer, ViiV Healthcare:[2]
- abacavir (ABC)
- lamivudine (3TC)
- zidovudine (AZT or ZDV)
 | |
| Combination of | |
|---|---|
| Abacavir | Nucleoside analogue reverse transcriptase inhibitor |
| Lamivudine | Nucleoside analogue reverse transcriptase inhibitor |
| Zidovudine | Nucleoside analogue reverse transcriptase inhibitor |
| Clinical data | |
| Trade names | Trizivir |
| AHFS/Drugs.com | FDA Professional Drug Information |
| MedlinePlus | a687007 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| KEGG | |
| NIAID ChemDB | |
| | |
It is indicated in the treatment of AIDS/HIV.[3] For this purpose, the combination is very useful in pregnant women to decrease the risk of mother-to-child transmission.[4]
The combination of drugs helps to reduce HIV's resistance (through mutation) to the drugs individually. Of the three, AZT and ABC have passed out of United States patent protection.
In December 2013, Lupin Limited launched a generic version of Trizivir.[5][6] As of 2016 the wholesale cost for a typical month of medication in the United States is more than 669.30 USD.[7]
References
- Opravil, Milos; Hirschel, Bernard; Lazzarin, Adriano; Furrer, Hansjakob; Chave, Jean‐Philippe; Yerly, Sabine; Bisset, Leslie R.; Fischer, Marek; Vernazza, Pietro; Bernasconi, Enos; Battegay, Manuel; Ledergerber, Bruno; Günthard, Huldrych; Howe, Colin; Weber, Rainer; Perrin, Luc (May 2002). "A Randomized Trial of Simplified Maintenance Therapy with Abacavir, Lamivudine, and Zidovudine in Human Immunodeficiency Virus Infection". The Journal of Infectious Diseases. 185 (9): 1251–1260. doi:10.1086/340312. PMID 12001042.
- InPharm.com: GlaxoSmithKline-Pfizer launch HIV joint venture Archived January 4, 2012, at the Wayback Machine
- Drugs.com: Trizivir
- Horvath, Tara; Madi, Banyana C; Iuppa, Irene M; Kennedy, Gail E; Rutherford, George W; Read, Jennifer S.; Horvath, Tara (2009). "Interventions for preventing late postnatal mother-to-child transmission of HIV". Cochrane Database Syst Rev (1): CD006734. doi:10.1002/14651858.CD006734.pub2. PMID 19160297.
- Monthly Prescribing Reference (MPR)
- Lupin Pharma
- "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Retrieved 12 December 2016.