Topotecan
Topotecan (trade name Hycamtin) is a chemotherapeutic agent that is a topoisomerase inhibitor. It is a synthetic, water-soluble analog of the natural chemical compound camptothecin. It is used in the form of its hydrochloride salt to treat ovarian cancer, lung cancer and other cancer types.
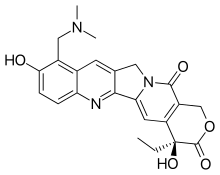 | |
| Clinical data | |
|---|---|
| Trade names | Hycamtin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610007 |
| License data | |
| Pregnancy category | |
| Routes of administration | Intravenous infusion, oral (capsules) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 31.4 % in humans[1][2] |
| Protein binding | 35% |
| Metabolism | Hepatic |
| Elimination half-life | 2–3 hours |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.213.372 |
| Chemical and physical data | |
| Formula | C23H23N3O5 •HCl |
| Molar mass | 421.45 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
After GlaxoSmithKline received final FDA approval for Hycamtin Capsules on October 15, 2007, topotecan became the first topoisomerase I inhibitor for oral use.
Uses
- Ovarian cancer (FDA May 1996).[3]
- Cervical cancer (FDA June 2006).[4][5]
- Small cell lung carcinoma (SCLC) (FDA Oct 2007).[6][7]
Experimental uses
As of 2016 experiments were under way for Neuroblastoma, Brainstem glioma, Ewing's sarcoma and Angelman's syndrome. In addition, topotecan is experimentally treating Non-small cell lung cancer, Colorectal Cancer, Breast cancer, Non-Hodgkin Lymphoma, Endometrial cancer, and Oligodendroglioma.[8]
Angelman's syndrome
Angelman’s syndrome is a neuro-genetic disorder characterized by severe developmental delays, seizures, speech impairments and physical impairments. It is an epigenetic disease and other treatments focus on symptoms. It is caused by a deletion or mutation of the maternal allele for the ubiquitin protein ligase E3A.[9] UBE3A is expressed in most body tissues. However, in neurons only the maternal copy of the gene is expressed. UBE3A is located on chromosome 15 and the paternal copy for the gene is genetically imprinted and is silenced by an antisense RNA transcript. The maternal copy control center of the gene is methylated, suppressing transcription in the antisense direction while the paternal copy control center is unmethylated.[10]
Treatment involves unsilencing the paternal allele allowing the normal paternal UBE3A allele to be transcribed. UBE3A, in normal function, adds ubiquitin chains to proteins to target unnecessary or damaged proteins for degradation by the proteasome.[11]
16 topoisomerase inhibitors unsilence paternal UBE3A. Topoisomerases are enzymes that regulate the unwinding of DNA.[12] Of these 16 inhibitors, topotecan was found to induce the strongest upregulation of UBE3A.[13] The enzymes bind to the DNA and cut the phosphate backbone, allowing the DNA to be unwound. Topotecan unsilences the paternal UBE2A allele by reducing the transcription of an antisense transcript. Topotecan inhibits topoisomerase I restoring UBE3A levels to wild-type range in cultured mince neurons.[14]
Transgenic mice with a fluorescently tagged UBE3A were used to test the effectiveness of unsilencing the paternal copy.[10] When tested on mice in vivo, topotecan affected the hippocampus, striatum and cerebral cortex but not the cerebellum unless a higher dose was administered (21.6 micrograms/hour for five days). The study suggested that the topoisomerase inhibitors have the potential to produce a normally functioning UBE3A protein. Most symptoms due to Angelman’s syndrome are traditionally treated by speech therapy, physical therapy and occupational therapy. Anti-seizure medication is often prescribed as seizures are a common symptom of Angelman’s syndrome.[15] These treatments target only symptoms.
This drug has been administered to cancer patients. It was well-tolerated when administered to pediatric and adult patients.
Mechanism of action
Topotecan is a semi-synthetic derivative of camptothecin. Camptothecin is a natural product extracted from the bark of the tree Camptotheca acuminata. Topoisomerase-I is a nuclear enzyme that relieves torsional strain in DNA by opening single strand breaks.[16] Once topoisomerase-I creates a single strand break, the DNA can rotate in front of the advancing replication fork. In physiological environments, topotecan is in equilibrium with its inactive carboxylate form.[17] Topotecan's active lactone form intercalates between DNA bases in the topoisomerase-I cleavage complex.[18] The binding of topotecan in the cleavage complex prevents topoisomerase-I from religating the nicked DNA strand after relieving the strain.[18] This intercalation therefore traps the topoisomerase-I in the cleavage complex bound to the DNA.[18] When the replication-fork collides with the trapped topoisomerase-I, DNA damage occurs.[18] The unbroken DNA strand breaks and mammalian cells cannot efficiently repair these double strand breaks.[19] The accumulation of trapped topoisomerase-I complexes is a known response to apoptotic stimuli.[20] This disruption prevents DNA replication and ultimately leads to cell death. This process leads to breaks in the DNA strand resulting in apoptosis. Administration of topotecan down-regulates its target, topoisomerase-I; therefore, it is dosed to maximize efficacy and minimize related toxicity.[17] Topotecan is often given in combination with Paclitaxel as first line treatment for extensive-stage small-cell lung cancer.[17]
Side effects
- Myelosuppression, specifically neutropenia, leukopenia, anemia, and thrombocytopenia
- Diarrhea, nausea, vomiting, stomatitis, and constipation
- Increased susceptibility to infections
- Asthenia[17]
Generic versions
Two generic versions have been approved in the European Union. In Nov 2010 the US FDA approved a generic version.[21][22]
References
- Leger, F.; Loos, W.; Bugat, R.; Mathijssen, R.; Goffinet, M.; Verweij, J.; Sparreboom, A.; Chatelut, E. (2004). "Mechanism-based models for topotecan-induced neutropenia". Clinical Pharmacology & Therapeutics. 76 (6): 567–578. doi:10.1016/j.clpt.2004.08.008. PMID 15592328.
- "Abstracts".
- "Archived copy". Archived from the original on January 19, 2009. Retrieved February 7, 2009.CS1 maint: archived copy as title (link)
- "FDA Approval for Topotecan Hydrochloride". National Cancer Institute.
- "Archived copy". Archived from the original on November 7, 2008. Retrieved February 7, 2009.CS1 maint: archived copy as title (link)
- "Archived copy". Archived from the original on June 26, 2009. Retrieved February 7, 2009.CS1 maint: archived copy as title (link)
- "GSK Receives Approval for Hycamtin (topotecan) Capsules for theTreatment of Relapsed Small Cell Lung Cancer".
- Haglof, K (2006). "Recent developments in the clinical activity of topoisomerase-1 inhibitors". Update on Cancer Therapeutics. 1 (2): 117–145. doi:10.1016/j.uct.2006.05.010.
- (Huang et al)
- Beaudet 2011.
- (Malzac)
- (Miller)
- (Malpass)
- (Huang)
- (Aditi and Williams)
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. (2010). "DNA topoisomerases and their poisoning by anticancer and antibacterial drugs". Chem. Biol. 17 (5): 421–433. doi:10.1016/j.chembiol.2010.04.012. PMID 20534341.
- Cordell, Geoffrey, ed. (2003). The Alkaloids: Chemistry and Biology. California: Academic Press. pp. 1–50. ISBN 978-0080521497.
- Pommier, Yves (2006). "Topoisomerase I inhibitors: camptothecins and beyond". Nature Reviews Cancer. 6 (10): 789–802. doi:10.1038/nrc1977. PMID 16990856.
- Staker, B.L.; et al. (2002). "The mechanism of topoisomerase I poisoning by a camptothecin analog". PNAS. 99 (24): 15387–15392. doi:10.1073/pnas.242259599. PMC 137726. PMID 12426403.
- Bertrand, Richard; Solary, Eric; O'Connor, Patrick; Kohn, Kurt W.; Pommier, Yves (1994-04-01). "Induction of a Common Pathway of Apoptosis by Staurosporine". Experimental Cell Research. 211 (2): 314–321. doi:10.1006/excr.1994.1093. PMID 8143779.
- "FDA Rubber-Stamps APP Pharma's Generic Topotecan for Small Cell Lung and Cervical Cancers". 30 Nov 2010.
- DNA Topoisomerases and Cancer, Yves Pommier Editor, Humana Press 2012
Sources
- Dagli, Aditi I.; Mueller, Jennifer; Williams, Charles A. (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora JH; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Angelman Syndrome. Seattle (WA): University of Washington, Seattle. PMID 20301323.
- Bailus, Barbara J.; Segal, David J. (2014-01-01). "The prospect of molecular therapy for Angelman syndrome and other monogenic neurologic disorders". BMC Neuroscience. 15: 76. doi:10.1186/1471-2202-15-76. ISSN 1471-2202. PMC 4069279. PMID 24946931.
- Beaudet, Arthur L. (2011-12-21). "Drugs to awaken a paternal gene". Nature. 481 (7380): 150–152. doi:10.1038/nature10784. ISSN 0028-0836. PMC 3638729. PMID 22190038.