Belinostat
Belinostat (trade name Beleodaq, previously known as PXD101) is a histone deacetylase inhibitor drug developed by TopoTarget for the treatment of hematological malignancies and solid tumors.[2]
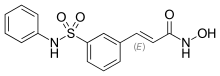 | |
| Clinical data | |
|---|---|
| Trade names | Beleodaq |
| Other names | PXD101 |
| AHFS/Drugs.com | beleodaq |
| Pregnancy category |
|
| Routes of administration | Intravenous (IV) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Protein binding | 92.9–95.8%[1] |
| Metabolism | UGT1A1 |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H14N2O4S |
| Molar mass | 318.348 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It was approved in July 2014 by the US FDA to treat peripheral T-cell lymphoma.[3]
In 2007 preliminary results were released from the Phase II clinical trial of intravenous belinostat in combination with carboplatin and paclitaxel for relapsed ovarian cancer.[4] Final results in late 2009 of a phase II trial for T-cell lymphoma were encouraging.[5] Belinostat has been granted orphan drug and fast track designation by the FDA,[6] and was approved in the US for the use against peripheral T-cell lymphoma on 3 July 2014.[3] It is not approved in Europe as of August 2014.[7]
The approved pharmaceutical formulation is given intravenously.[8]:180 Belinostat is primarily metabolized by UGT1A1; the initial dose should be reduced if the recipient is known to be homozygous for the UGT1A1*28 allele.[8]:179 and 181
References
- "Beleodaq (belinostat) For Injection, For Intravenous Administration. Full Prescribing Information" (PDF). Spectrum Pharmaceuticals, Inc. Irvine, CA 92618. Retrieved 21 November 2015.
- Plumb JA; Finn PW; Williams RJ; et al. (2003). "Pharmacodynamic Response and Inhibition of Growth of Human Tumor Xenografts by the Novel Histone Deacetylase Inhibitor PXD101". Molecular Cancer Therapeutics. 2 (8): 721–728. PMID 12939461.
- "FDA approves Beleodaq to treat rare, aggressive form of non-Hodgkin lymphoma". FDA. 3 July 2014.
- "CuraGen Corporation (CRGN) and TopoTarget A/S Announce Presentation of Belinostat Clinical Trial Results at AACR-NCI-EORTC International Conference". October 2007. Archived from the original on 2011-07-16. Retrieved 2011-12-06.
- Final Results of a Phase II Trial of Belinostat (PXD101) in Patients with Recurrent or Refractory Peripheral or Cutaneous T-Cell Lymphoma, December 2009
- "Spectrum adds to cancer pipeline with $350M deal". February 2010.
- H. Spreitzer (4 August 2014). "Neue Wirkstoffe – Belinostat". Österreichische Apothekerzeitung (in German) (16/2014): 27.
- Lexicomp, (corporate author) (2016). Bragalone, DL (ed.). Drug Information Handbook for Oncology (14th ed.). Wolters Kluwer. ISBN 9781591953517.