Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone, however refers to the isomer, 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Anthraquinone | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.408 |
| KEGG | |
CompTox Dashboard (EPA) |
|
SMILES
| |
| Properties | |
Chemical formula |
C14H8O2 |
| Molar mass | 208.216 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.308 g/cm3 |
| Melting point | 286 °C (547 °F; 559 K) |
| Boiling point | 379.8 °C (715.6 °F; 653.0 K) |
Solubility in water |
insoluble |
| Hazards | |
| R-phrases (outdated) | R36/37/38 |
| Flash point | 185 °C (365 °F; 458 K) |
| Related compounds | |
Related compounds |
quinone, anthracene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
There are several current industrial methods to produce 9,10-Anthraquinone:
- The oxidation of anthracene, a reaction that is localized at the central ring. Chromium(VI) is the typical oxidant.
- The Friedel-Crafts reaction of benzene and phthalic anhydride in presence of AlCl3 producing o-benzoylbenzoic acid which then undergoes cyclization, forming anthraquinone. This reaction is useful for producing substituted anthraquinones.
- The Diels-Alder reaction of naphthoquinone and butadiene followed by oxidative dehydrogenation
- The acid-catalyzed dimerization of styrene to give a 1,3-diphenylbutene, which then can be transformed to the anthraquinone.[1] This process was pioneered by BASF.
It also arises via the Rickert-Alder reaction, a retro-Diels-Alder reaction.
Applications and natural occurrence
Dyestuff precursor
 The yellow color of certain lichens (here Caloplaca thallincola) is due to the présence of anthraquinones.
The yellow color of certain lichens (here Caloplaca thallincola) is due to the présence of anthraquinones.
Synthetic dyes are often derived from 9,10-anthraquinone, such as alizarin.[2] Important derivatives are 1-nitroanthraquinone, anthraquinone-1-sulfonic acid, and the dinitroanthraquinone.[1] Natural pigments that are derivatives of anthraquinone are found, inter alia, in aloe latex, senna, rhubarb, and cascara buckthorn, fungi, lichens, and some insects.
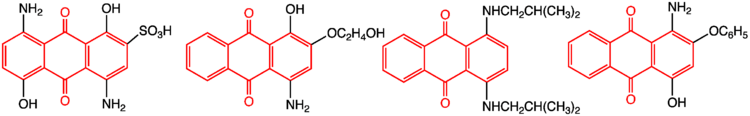
Digester additive in papermaking
9,10-Anthraquinone is used as a digester additive in production of paper pulp by alkaline processes, like the Kraft, the alkaline sulfite or the Soda-AQ processes. The anthraquinone is a redox catalyst. The reaction mechanism may involve single electron transfer (SET).[3] The anthraquinone is oxidizing the reducing end of polysaccharides in the pulp, i.e., cellulose and hemicellulose, and thereby protecting it from alkaline degradation (peeling). The anthraquinone is reduced to 9,10-dihydroxyanthracene which then can react with lignin. The lignin is degraded and becomes more watersoluble and thereby more easy to wash away from the pulp, while the antraquinone is regenerated. This process gives an increase in yield of pulp, typically 1-3% and a reduction in kappa number.[4]
Sodium 2-anthraquinonesulfonate (AMS) is a watersoluble anthraquinone derivative that was the first anthraquinone derivative discovered to have a catalytic effect in the alkaline pulping processes.[5]
In the production of hydrogen peroxide
A large industrial application of anthraquinones is for the production of hydrogen peroxide. 2-Ethyl-9,10-anthraquinone or a related alkyl derivative is used, rather than anthraquinone itself.[6]
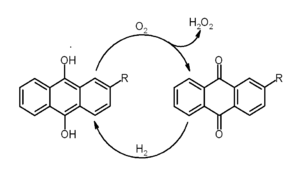 Catalytic hydrogen peroxide production with the anthraquinone process
Catalytic hydrogen peroxide production with the anthraquinone process
Medicine
Derivatives of 9,10-anthraquinone include many important drugs (collectively called anthracenediones). They include
- Laxatives such as dantron, emodin, and aloe emodin, and some of the senna glycosides
- Antimalarials such as rufigallol
- Antineoplastics used in the treatment of cancer, such as mitoxantrone, pixantrone, and the anthracyclines
- DNA dyes / nuclear counterstains such as DRAQ5, DRAQ7 and CyTRAK Orange for flow cytometry and fluorescence microscopy.
- Anthraquinone derivatives: rhein, emodin, aloe emodin, parietin (physcion), and chrysophanol extracted from Cassia occidentalis are toxic and known to cause hepatomyoencephalopathy in children.[7]
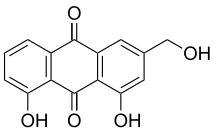 Aloe emodin
|
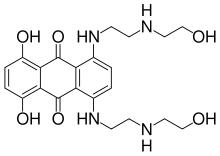 Mitoxantrone
|
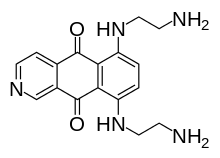 Pixantrone
|
Niche uses
9,10-Anthraquinone is used as a bird repellant on seeds, and as a gas generator in satellite balloons.[8] It has also been mixed with lanolin and used as a wool spray to protect sheep flocks against kea attacks in New Zealand.[9]
Natural anthraquinone derivatives tend to have laxative effects. Prolonged use and abuse leads to melanosis coli.[10][11] 5 anthraquinones have been shown to inhibit the formation of Tau aggregates and dissolve paired helical filaments thought to be critical to Alzheimer's disease progression in both mouse models and in vitro testing but have not been investigated as a therapeutic agent.[12]
In 2019 researchers pioneered the possible use of the material as a cathode in a novel aluminum ion battery.[13]
Other isomers
Several other isomers of anthraquinone are possible, including the 1,2-, 1,4-, and 2,6-anthraquinones. They are of comparatively minor importance. The term is also used in the more general sense of any compound that can be viewed as an anthraquinone with some hydrogen atoms replaced by other atoms or functional groups. These derivatives include substances that are technically useful or play important roles in living beings.
Recently, a class of anthraquinone derivates were shown to have self-healing properties when doped in PMMA matrix.[14]
Metabolism in humans
The enzyme encoded by the gene UGT1A8 has glucuronidase activity with many substrates including anthraquinones.[15]
Honeycomb structure on Cu(111)
Anthraquinone molecules adsorbed onto a Cu(111) surface assemble into a highly ordered honeycomb structure.[16] The self-assembly of organic molecules onto metal surfaces is a frequently occurring phenomenon. However, the honeycomb structure formed by anthraquinone is unique in its unusually large pore diameter (≈5 nm). Additionally, the patterned structure of anthraquinone is supported theoretically using both classical Monte Carlo[17] and quantum mechanical DFT[18] approaches.
See also
- Benzoquinone
- Naphthoquinone
- Parietin
References
- Vogel, A. "Anthraquinone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_347.
- Bien, H.-S.; Stawitz,, J.; Wunderlich, K. (2005). "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.CS1 maint: extra punctuation (link)
- Samp, J. C. (2008). "A comprehensive mechanism for anthraquinone mass transfer in alkaline pulping". Georgia Institute of Technology: 30. Cite journal requires
|journal=(help) - Sturgeoff, L. G.; Pitl, Y. (1997) [1993]. "Low Kappa Pulping without Capital Investment". In Goyal, G. C. (ed.). Antraquinone Pulping. TAPPI Press. pp. 3–9. ISBN 0-89852-340-0.
- "Anthraquinone / Alkali Pulping - A Literature Review" (pdf). Project 3370. Appleton, Wisconsin: The Institute of Paper Chemistry. 1978-07-05.
- Goor, G.; Glenneberg, J.; Jacobi, S. (2007). "Hydrogen Peroxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_443.pub2.
- Panigrahi, G.K.; Suthar, M.K.; Verma, N.; Asthana, S.; Tripathi, A.; Gupta, S.K.; Saxena, J. K.; Raisuddin, S.; Das, M. (2015). "Investigation of the interaction of anthraquinones of Cassia occidentalis seeds with bovine serum albumin by molecular docking and spectroscopic analysis: Correlation to their in vitro cytotoxic potential". Food Research International. doi:10.1016/j.foodres.2015.08.022.
- "www.americanheritage.com". Archived from the original on 2009-06-09. Retrieved 2009-09-22.
- Dudding, Adam (29 July 2012). "How to solve a problem like a kea". Sunday Star Times. New Zealand. Retrieved 11 November 2014.
- Müller-Lissner, S. A. (1993). "Adverse Effects of Laxatives: Fact and Fiction". Pharmacology. 47 (Suppl 1): 138–145. doi:10.1159/000139853. PMID 8234421.
- Moriarty, K. J.; Silk, D. B. (1988). "Laxative Abuse". Digestive Diseases. 6 (1): 15–29. doi:10.1159/000171181. PMID 3280173.
- Pickhardt, M.; Gazova, Z.; von Bergen, M.; Khlistunova, I.; Wang, Y.; Hascher, A.; Mandelkow, E. M.; Biernat, J.; Mandelkow, E. (2005). "Anthraquinones Inhibit Tau Aggregation and Dissolve Alzheimer's Paired Helical Filaments in vitro and in Cells" (pdf). The Journal of Biological Chemistry. 280 (5): 3628–3635. doi:10.1074/jbc.M410984200. PMID 15525637.
- Paraskova, Tsvetana (October 1, 2019). "Is This The End Of The Lithium-Ion Battery?". OilPrice.com. Retrieved 2019-10-06.
- Ramini, Shiva K.; Kuzyk, Mark G. (2012-08-07). "A self healing model based on polymer-mediated chromophore correlations". The Journal of Chemical Physics. 137 (5): 054705. arXiv:1205.0481. doi:10.1063/1.4739295. ISSN 0021-9606.
- Ritter, J. K.; Chen, F.; Sheen, Y. Y.; Tran, H. M.; Kimura, S.; Yeatman, M. T.; Owens, I. S. (1992). "A Novel Complex Locus UGT1 Encodes Human Bilirubin, Phenol, and other UDP-Glucuronosyltransferase Isozymes with Identical Carboxyl Termini" (pdf). Journal of Biological Chemistry. 267 (5): 3257–3261. PMID 1339448.
- Pawin, G.; Wong, K. L.; Kwon, K.; Bartels, L. (2006). "A Homomolecular Porous Network at a Cu(111)". Science. 313: 961–962. doi:10.1126/science.1129309.
- Šimėnas, M.; Tornau, E. E. (2013). "Pin-wheel hexagons: A model for anthraquinone ordering on Cu(111)". J. Chem. Phys. 139: 154711. doi:10.1063/1.4825079.
- Wyrick, J.; et al. (2011). "Do Two-Dimensional "Noble Gas Atoms" Produce Molecular Honeycombs at a Metal Surface?". Nano Lett. 11 (7): 2944–2948. doi:10.1021/nl201441b.