Rucaparib
Rucaparib (brand name Rubraca /ruːˈbrɑːkə/ roo-BRAH-kə) is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California.[2] It is being developed by Clovis Oncology.
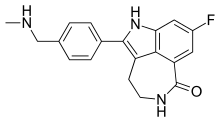 | |
| Clinical data | |
|---|---|
| Pronunciation | /ruːˈkæpərɪb/ roo-KAP-ər-ib |
| Trade names | Rubraca |
| Other names | AG014699 |
| AHFS/Drugs.com | rubraca |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30–45% (Tmax = 1.9 hours) |
| Protein binding | 70% (in vitro) |
| Metabolism | Liver (primarily CYP2D6; 1A2 and 3A4 to a lesser extent) |
| Elimination half-life | 17–19 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.490 |
| Chemical and physical data | |
| Formula | C19H18FN3O |
| Molar mass | 323.371 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
In December 2016, the U.S. FDA granted an accelerated approval for use in cases of pretreated advanced ovarian cancer.[3]
In Europe it was designated as an orphan medicinal product on 10 October 2012. On 22 March 2018 the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a conditional marketing authorisation, intended for the treatment of relapsed or progressive ovarian cancer.[4]
Pharmacology
Mechanism of action
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture."[6]
As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).[7]
Regulatory information
Clinical trials
In 2013, a total of 106 patients from six different countries were enrolled into phase II trial (ARIEL2) to evaluate the activity of the investigational drug in women with platinum-sensitive advanced ovarian, fallopian tube, or primary peritoneal cancer. By 2015, the initial data from ARIEL2 were presented at ASCO, representing breakthrough therapy designation. of 106 patients in the study, 52 of the subjects did not show any adverse event, such as death or disease progression. Of these 52 patients, 18 subjects discontinued treatment for personal reasons.[8]
In the first quarter of 2016, the marketing applications were submitted in order to prove of the treatment of advanced ovarian cancer in women with deleterious BRCA mutation–positive (BRCAmut+). In June 2016, an NDA was filed with the FDA and the PDUFA date assigned for February 2017. However, surprisingly, rucaparib was granted fast-track status and was approved by the FDA in December 2016 as a monotherapy treatment of mentioned patients who have been treated at least two prior chemotherapies. Furthermore, the FDA also approved the FoundationFocus CDxBRCA Test as the first next-generation sequencing (NGS)-based companion diagnostic to identify the most potent ovarian cancer patients who responded to rucaparib therapy.[9]
After the FDA approval, TRITON2 and TRITON3 mCRPC studies were initiated in order to determine how patients with prostate cancer will respond to the rucaparib drug. The studies for these two trials are still going on and the estimated dates for the first results are range between 2019 and 2022.[10]
The ARIEL3 and ARIEL4 are two randomized, double-blind phase III studies. The ARIEL3 study was designed to evaluate the effect of the investigational agent as a maintenance treatment for the advanced platinum-sensitive ovarian cancer patients compared placebo after their response to at least two prior chemotherapies. The top-line results from the study were presented at the ESMO 2017 congress and right after that, it was published in the Lancet journal in September 2017. The findings showed significant improvement in progression-free survival (PFS) in patients treated with Rubraca than placebo. Recently, in October 2017, a supplemental sNDA for the rucaparib ARIEL3 maintenance treatment has been submitted to the FDA.[11]
The ARIEL4 trial is still ongoing to evaluate how patients will best respond to treatment with rucaparib compared with chemotherapy. The estimated data collection date for primary outcome measurement will be in June 2022.[12]
Commercialization
Rucaparib is a prescription medicine being commercialized under the brand name Rubraca® by a biopharmaceutical company called Clovis Oncology, Inc. (NASDAQ: CLVS). The highest goal of the company is the discovery, development, and commercialization of novel anti-tumour drugs, including Rubraca.[13]
Commercial aspects
Clovis has reported a significant net loss since its inception, but during 2017 they had a transition to profitability relying on the revenues from their only marketed drug, Rubraca. In January 2017, the company has sold 5,750,000 shares ($41.00 per share) and their net proceeds were $221.2 million. Moreover, in June 2017 they sold 3,920,454 shares ($88.00 per share) with net proceeds of $324.9 million. The goal of the company is to spend the mentioned net proceeds of the offering for marketing and sales expenses associated with Rubraca.[14]
In June 2011, Pfizer announced an agreement with Clovis Oncology Inc. that permits the company to defer the milestone payments payable upon regulatory approval of an NDA. The Clovis has also been licensed from AstraZeneca in April 2012 to develop the rucaparib medicine for certain methods of treatment of patients with PARP inhibitors. In the first quarter of 2017, Clovis paid $0.75 million milestone payment to Pfizer. The company has also been committed to pay a $20.0 million milestone payment upon the FDA approval. However, Clovis has agreed to pay $3.0 million extra within 18 months in the hope of making a significant profit by selling Rubraca.[15]
Intellectual property
There are a total of eight patents from the US Patent and Trademark Office (USPTO) and one NDA for the marketed rucaparib.[16]
The patents are related to the production methods, using methods, high dosage strength tablets, formulations, and multiple salt/polymorphic forms of the drug, with expiration dates between 2022 and 2035. For instance, the patent relating to the composition of matter will expire in 2020 and the camsylate salt/polymorph patent family which is licensed from Pfizer will expire in 2031. Additional patent applications are still pending in the United States or Europe in various jurisdictions that, if issued, would have expiration dates ranging from 2029 through 2033.[17]
See also
References
- "Rubraca (rucaparib) Tablets, for Oral Use. Full Prescribing Information" (PDF). Clovis Oncology, Inc. Boulder, CO 80301. Retrieved 20 December 2016.
- White, AW; Almassy, R; Calvert, AH; Curtin, NJ; Griffin, RJ; Hostomsky, Z; Maegley, K; Newell, DR; Srinivasan, S; Golding, BT (2 November 2000). "Resistance-Modifying Agents. 9. Synthesis and Biological Properties of Benzimidazole Inhibitors of the DNA Repair Enzyme Poly(ADP-ribose) Polymerase". Journal of Medicinal Chemistry. 43 (22): 4084–97. doi:10.1021/jm000950v. PMID 11063605.
- Bankhead, C (December 19, 2016). "PARP Inhibitor Gets FDA Nod for Ovarian Cancer". MedPage Today, LLC. Retrieved 20 December 2016.
- http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004272/smops/Positive/human_smop_001275.jsp&mid=WC0b01ac058001d127
- "Cancer Research Launches New Drug Trial". netdoctor.co.uk. Hearst Magazines UK. 2012-01-10. Retrieved 20 December 2016.
- "Archived copy" (PDF). Archived from the original (PDF) on 2011-06-13. Retrieved 2009-11-17.CS1 maint: archived copy as title (link)
- "Rucaparib shows clinical benefit in pancreatic cancer patients with BRCA mutation: Results suggest a potential -- and much-needed -- treatment option for some pancreatic cancer patients". sciencedaily.com.
- "Clovis Oncology Presents Data from Phase 2 Studies of Rucaparib in Advanced Ovarian Cancer and Pancreatic Cancer at 2016 ASCO Annual Meeting",http://www.businesswire.com/news/home/20160606005594/en/Clovis-Oncology-Presents-Data-Phase-2-Studies
- "Rucaparib", https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm533891.htm
- "CURRENT CLOVIS CLINICAL TRIALS INVESTIGATING RUCAPARIB", http://clovisoncology.com/files/Clovis_Oncology_Clinical_Trial_Fact_Sheet_FINAL_1.pdf
- Coleman, Robert L.; Oza, Amit M.; Lorusso, Domenica; Aghajanian, Carol; Oaknin, Ana; Dean, Andrew; Colombo, Nicoletta; Weberpals, Johanne I.; Clamp, Andrew; Scambia, Giovanni; Leary, Alexandra; Holloway, Robert W.; Gancedo, Margarita Amenedo; Fong, Peter C.; Goh, Jeffrey C.; O'Malley, David M.; Armstrong, Deborah K.; Garcia-Donas, Jesus; Swisher, Elizabeth M.; Floquet, Anne; Konecny, Gottfried E.; McNeish, Iain A.; Scott, Clare L.; Cameron, Terri; Maloney, Lara; Isaacson, Jeff; Goble, Sandra; Grace, Caroline; Harding, Thomas C.; et al. (2017). "Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial" (PDF). The Lancet. 390 (10106): 1949–1961. doi:10.1016/S0140-6736(17)32440-6. PMC 5901715. PMID 28916367.
- "CLOVIS ONCOLOGY TO PRESENT COMPREHENSIVE DATASET FROM SUCCESSFUL ARIEL3 CLINICAL TRIAL PROGRAM AT 2017 ESMO CONGRESS",http://ir.clovisoncology.com/phoenix.zhtml?c=247187&p=irol-newsArticle&ID=2297323
- "Rucaparib: First Global Approval" ,Drugs, Yahiya Y. Syed, 2017, Volume 77, P. 585
- "Clovis Oncology (CLVS)FORM 10-Q" ,https://www.sec.gov/Archives/edgar/data/1466301/000155837017005797/clvs-20170630x10q.htm
- "Financial Reports SEC Filings" ,http://phx.corporate-ir.net/phoenix.zhtml?c=247187&p=irol-sec
- "What is the patent landscape for Rubraca, and what generic Rubraca alternatives are available?" ,https://www.drugpatentwatch.com/p/tradename/RUBRACA
- "CLOVIS ONCOLOGY, INC. filed this Form S-3ASR on 01/03/2017" ,http://ir.clovisoncology.com/mobile.view?c=247187&v=202&d=3&id=aHR0cDovL2FwaS50ZW5rd2l6YXJkLmNvbS9maWxpbmcueG1sP2lwYWdlPTExMzAxODk1JkRTRVE9MSZTRVE9NyZTUURFU0M9U0VDVElPTl9QQUdFJmV4cD0mc3Vic2lkPTU3