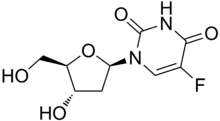
Floxuridine
Floxuridine (also 5-fluorodeoxyuridine) is an oncology drug that belongs to the class known as antimetabolites. Specifically, floxuridine is a pyrimidine analog, classified as a deoxyuridine.[1] The drug is usually administered via an artery, and most often used in the treatment of colorectal cancer. The quality of life and survival rates of individuals that receive continuous hepatic artery infusion of floxuridine for colorectal cancer metastases is significantly higher than control groups.[2] Floxuridine can also be prescribed for the treatment of kidney and stomach cancers.[3] In vitro uses of floxuridine include 5-minute treatments of fluorouracil, floxuridine, and mitomycin to increase cell proliferation in Tenon's capsule fibroblasts.[4]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682006 |
| Pregnancy category |
|
| Routes of administration | Intra-arterial |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.066 |
| Chemical and physical data | |
| Formula | C9H11FN2O5 |
| Molar mass | 246.194 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 150.5 °C (302.9 °F) |
SMILES
| |
InChI
| |
| (verify) | |
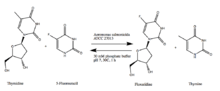
Biosynthesis
Immobilized Aeromonas salmonicida ATCC 27013, when exposed to thymidine and 5-fluorouracil in phosphate buffer at room temperature for one hour, can synthesize floxuridine and thymine.[5]
Pharmacology[6]
Floxuridine primarily works by stopping the growth of newly born cells. The drug essentially stops DNA from forming in new and rapidly developing cells, which is a sign of a cancerous cell. Therefore, the floxuridine kills the cancerous cells. For colorectal cancer and hepatic metastases, an average adult should be given an Intra-arterial dosage of 0.1–0.6 mg/kg/day as a continuous infusion, continued until intolerable toxicity is reached (white blood cell count <3,500/mm^3 or platelet count <100,000/mm^3).[7] Lethal dosages for other species are below.[8] LD50 is the lethal dose at which half of organisms exposed to the drug die.
| Species | LD50 (mg/kg +/- SE) |
|---|---|
| Mouse | 880 +/- 51 |
| Rat | 670 +/- 73 |
| Rabbit | 94 +/- 19.6 |
| Dog | 157 +/- 46 |
Pharmacodynamics
Floxuridine is a pyrimidine analog that acts as an inhibitor of the S-phase of cell division. This selectively kills rapidly dividing cells. Antimetabolites masquerade as pyrimidine-like molecules which prevents normal pyrimidines from being incorporated into DNA during the S phase of the cell cycle. Fluorouracil (the end-product of catabolism of floxuridine) blocks an enzyme which converts cytosine nucleosides into the deoxy derivative. In addition, DNA synthesis is further inhibited because fluoruracil blocks the incorporation of the thymidine nucleotide into the DNA strand.
Mechanism of action
Floxuridine is rapidly catabolized to 5-fluorouracil, which is the active form of the drug. The primary effect is interference with DNA synthesis and to a lesser extent, inhibition of RNA formation through the drug's incorporation into RNA, thus leading to the production of fraudulent RNA. Fluorouracil also inhibits uracil riboside phosphorylase, which prevents the utilization of preformed uracil in RNA synthesis. As well, the monophosphate of floxuridine, 5-fluoro-2'-deoxyuridine-5'-phosphate (FUDR-MP) inhibits the enzyme thymidylate synthetase. This leads to the inhibition of methylation of deoxyuridylic acid to thymidylic acid, thus interfering with DNA synthesis.
Route of Elimination
The drug is excreted intact and as urea, fluorouracil, α-fluoro-β-ureidopropionic acid, dihydrofluorouracil, α-fluoro-β-guanidopropionic acid and α-fluoro-β-alanine in the urine; it is also expired as respiratory carbon dioxide.
Side effects
Side effects include:[9]
Common (30% of patients)
- Low blood counts. Your white and red blood cells and platelets may temporarily decrease. This can put you at increased risk for infection, anemia and/or bleeding.
- Mouth sores
- Diarrhea (may be severe)
Less common (10–29% of patients)
- Poor appetite
- Nausea and vomiting
- Hair loss
- Elevated liver enzymes (temporary increase in alkaline phosphatase, lactate dehydrogenase, transaminase, and bilirubin). This is seen more with the intra-arterial infusion directly into the liver.
- Hand-foot syndrome (Palmar-plantar erythrodysesthesia or PPE) -skin rash, swelling, redness, pain and/or peeling of the skin on the palms of hands and soles of feet
- Stomach ulcers (This is seen more with the intra-arterial infusion).
Contact your health provider immediately
- Fever of 100.4 °F (38 °C) or higher, chills (possible signs of infection).
Contact your health provider
- Diarrhea (2 episodes in a 24-hour period)
- Nausea (interferes with ability to eat and unrelieved with prescribed medication)
- Vomiting (vomiting more than 4–5 times in a 24-hour period)
- Mouth sores (painful redness, swelling or ulcers)
- Unusual bleeding or bruising
- Black or tarry stools, or blood in your stools
- Blood in the urine
- Yellowing of the skin or eyes
- Tingling or burning, redness, swelling of the palms of the hands or soles of feet
Other
- Fertility for both men and women may be affected by floxuridine.
History
Floxuridine first gained FDA approval in December 1970 under the brand name FUDR. The drug was initially marketed by Roche, which also did a lot of the initial work on 5-fluorouracil. The National Cancer Institute was an early developer of the drug. Roche sold its FUDR product line in 2001 to F H Faulding, which became Mayne Pharma.
Alternative names
Synonyms for floxuridine include:[10]
|
|
|
References
- "Floxuridine". PubChem. Retrieved 18 April 2017.
- Allen-Mersh, TG; Earlam, S; Fordy, C; Abrams, K; Houghton, J (November 1994). "Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases". The Lancet. 344 (8932): 1255–1260. doi:10.1016/S0140-6736(94)90750-1.
- "Floxuridine". Chemocare. Chemocare.com. Retrieved 17 April 2017.
- Khaw, Peng T.; Sherwood, Mark B.; Mackay, Sally L. D. (August 1992). "Five-Minute Treatments With Fluorouracil, Floxuridine, and Mitomycin Have Long-term Effects on Human Tenon's Capsule Fibroblasts". JAMA Ophthalmology. 110 (8): 1150. doi:10.1001/archopht.1992.01080200130040. Retrieved 7 May 2017.
- Rivero, Cintia; Britos, Claudia; Mario, Lozano; Sinisterra, Jose; Trelles, Jorge (March 12, 2012). "Green biosynthesis of floxuridine by immobilized microorganisms". FEMS Microbiology Letters. 331 (331): 31–36. doi:10.1111/j.1574-6968.2012.02547.x.
- Canadian Institutes of Health Research. "Floxuridine". DrugBank. Retrieved 18 April 2017.
- "Floxuridine". Drugs.com.
- "Floxuridine". Bedford Laboratories.
- "Floxuridine". Chemocare. Chemocare.com. Retrieved 17 April 2017.
- Canadian Institutes of Health Research. "Floxuridine". DrugBank. Retrieved 18 April 2017.