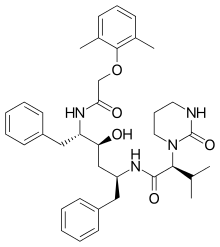
Lopinavir
Lopinavir is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir (lopinavir/ritonavir).[1]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | ABT-378 |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a602015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 98-99% |
| Metabolism | Hepatic |
| Elimination half-life | 5 to 6 hours |
| Excretion | Mostly fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C37H48N4O5 |
| Molar mass | 628.810 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It was patented in 1995 and approved for medical use in 2000.[2]
Side effects
Side effects, interactions, and contraindications have only been evaluated in the drug combination lopinavir/ritonavir.
Pharmacology
Lopinavir is highly bound to plasma proteins (98–99%).[3]
Reports are contradictory regarding lopinavir penetration into the cerebrospinal fluid (CSF). Anecdotal reports state that lopinavir cannot be detected in the CSF; however, a study of paired CSF-plasma samples from 26 patients receiving lopinavir/ritonavir found lopinavir CSF levels above the IC50 in 77% of samples.[4]
Research
A 2014 study indicates that lopinavir is effective against the human papilloma virus (HPV). The study used the equivalent of one tablet twice a day applied topically to the cervices of women with high-grade and low-grade precancerous conditions. After three months of treatment, 82.6% of the women who had high-grade disease had normal cervical conditions, confirmed by smears and biopsies.[5]
References
- "FDA Approved Drug Products: Kaletra". Retrieved 30 April 2004.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 510. ISBN 9783527607495.
- KALETRA (lopinavir/ritonavir) capsules; (lopinavir/ritonavir) oral solution. Prescribing information. April 2009
- Capparelli E, Holland D, Okamoto C, et al. (2005). "Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV". AIDS. 19 (9).
- HIV drug used to reverse effects of virus that causes cervical cancer University of Manchester, 17 February 2014.