Abacavir/dolutegravir/lamivudine
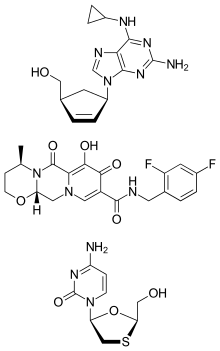
Abacavir/dolutegravir/lamivudine (brand name Triumeq) is a fixed-dose combination medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: 600 mg abacavir (reverse transcriptase inhibitor), 50 mg dolutegravir (integrase inhibitor) and 300 mg lamivudine (nucleoside analog reverse transcriptase inhibitor).
 | |
| Combination of | |
|---|---|
| Abacavir | nucleoside analog reverse transcriptase inhibitor |
| Dolutegravir | integrase inhibitor |
| Lamivudine | nucleoside analog reverse transcriptase inhibitor |
| Clinical data | |
| Trade names | Triumeq |
| AHFS/Drugs.com | https://www.drugs.com/mtm/triumeq.html |
| Pregnancy category |
|
| Routes of administration | oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| KEGG | |
The medication was developed by ViiV Healthcare and approved by the Food and Drug Administration for use in the United States in August 2014.[1]
Abacavir is a nucleotide reverse transcriptase inhibitor. Specifically, abacavir is a guanosine analogue that interferes with HIV viral RNA-dependent DNA polymerase, ultimately resulting in inhibition of replication of HIV. Dolutegravir inhibits the HIV replication cycle by binding to the integrase active site and inhibiting the strand transfer step of HIV-1 DNA integration. Lamivudine is a cytosine analogue that inhibits HIV reverse transcription by terminating the viral DNA chain.[2]
Research
Clinical trials
Efficacy of Triumeq was demonstrated in antiretroviral treatment-naive patients by SINGLE (ING114467), the randomized, controlled trial and other trials in treatment-naive subjects (see Tivicay, dolutegravir).
In the SINGLE trial, 414 patients received Tivicay (dolutegravir) + Epzicom (abacavir/lamivudine) once daily and 419 patients received Atripla (efavirenz/emtricitabine/tenofovir) once daily. Tivicay + Epzicom compared to Atripla showed a reduction in viral load of HIV-1 RNA <50 copies/mL in 80% of patients compared to 72% of patients, respectively. Furthermore, in patients with baseline plasma viral load of <100,000 and >100,000 copies/mL, Tivicay + Epzicom compared to Atripla showed a reduction to <50 copies/mL in 85% and 71% compared to 73% and 72%, respectively.[3]
Post-marketing experience
In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been reported voluntarily from a population of unknown size. As such, it is not always possible to estimate frequency or establish a causal relationship to drug exposure.[4]
Abacavir and/or Lamivudine
- Digestive System: stomatitis
- Gastrointentional: pancreatitis
- General: weakness
- Blood and Lymphatic Systems: aplastic anemia, anemia, enlarged lymph nodes, enlarged spleen
- Hypersensitivity: sensitization reactions (including anaphylaxis), urticaria
- Metabolism and Nutrition Disorders: hyperprolactinemia
- Musculoskeletal System: muscle weakness, CPK elevation, rhabdomyolysis
- Nervous System: paresthesia, peripheral neuropathy, seizures
- Respiratory System: abnormal breath sounds/wheezing
- Skin: hair loss, erythema multiforme. Suspected Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in patients receiving abacavir primarily in combination with medications that are known to be associated with SJS and TEN, respectively.
Adverse effects
The following adverse reactions were reported in <2% of patients:[2]
- Central nervous system: drowsiness, lethargy, nightmares, sleep disorders, suicidal ideation
- Dermatologic: pruritus
- Endocrine and metabolic: high levels of triglycerides
- Gastrointestinal: abdominal distention, abdominal distress, abdominal pain, lack of appetite, stomach upset, flatulence, gastroesophageal reflux disease, upper abdominal pain, vomiting
- Hepatic: hepatitis
- Neuromuscular and skeletal: joint pain, muscle inflammation
- Kidney: chronic kidney disease
- Miscellaneous: fever
See individual agents as well as other combination products for additional information.
Pregnancy
Triumeq is pregnancy category C as there are no adequate and well-controlled trials in pregnant women. However, reproduction studies with the components of Triumeq have been performed in animals. Triumeq should only be used in pregnancy if the potential benefits outweigh the risks. To monitor maternal-fetal outcomes of pregnant women that have used an antiretroviral, including Triumeq, an Antiretroviral Pregnancy Registry has been established.[5]
Breastfeeding
The Centers for Disease Control and Prevention (CDC) recommend that HIV-infected mothers do not breastfeed their infants to avoid risking postnatal transmission of HIV. This recommendation is coupled with the potential for serious adverse reactions in nursing infants. Dolutegravir and abacavir were shown to be excreted in the milk of lactating rats. Lamivudine was shown to be excreted in human breast milk.[4]
History
Approval
Patent was filed on April 28, 2006[6] and expires October 5, 2027.[7] Triumeq was approved by the Federal Drug and Food Administration (FDA) on August 22, 2014.
Recent major label changes[4]
In August 2015, the FDA sent a bulletin regarding label updates for Tivicay and Triumeq regarding drug-drug information.
Drug interactions was updated to include a statement that in vitro, dolutegravir was not a substrate of OATP1B1 or OATP1B3. Furthermore, information regarding drug interactions with carbamazepine and metformin.
Additionally, less common adverse reactions observed in clinical trials was updated to include suicidal ideation, attempt, behavior, or completion in order to be consistent with Tivicay label.[8]
In September 2015, the FDA added a boxed warning of hypersensitivity reactions, lactic acidosis, and severe hepatomegaly in abacavir-containing products regarding HLA-B*507 allele.[9]
Boxed warning (9/2015)
- Hypersensitivity reactions
- Lactic acidosis and severe hepatomegaly with steatosis
- Exacerbations of hepatitis B
Dosage and administration
- Dosage recommendation with certain concomitant medications (8/2015)
- Not recommended due to lack of dosage adjustment (9/2015)
Contraindications (9/2015)
Warnings and precautions, hypersensitivity reactions (9/2015)
Society and culture
Utilization
ViiV Healthcare is a conglomerate independent pharmaceutical company established by GlaxoSmithKline and Pfizer in 2009 with sole focus on HIV treatment and care. In 2012, Shionogi joined following a long-term collaboration in the joint development of several novel integrase inhibitors. In 2014, ViiV Healthcare's current 12 HIV treatments generated annual sales of about $2.3 billion.[10] Sales for GlaxoSmithKline were up 15% in 2014, following the launches of Tivicay and Triumeq (combined sales of $510 million).[11]
Other medications patented by ViiV Healthcare for HIV treatment include:[12]
- Tivicay (dolutegravir)
- Selzentry/Celsentri (maraviroc)
- Epzicom/Kivexa (abacavir/lamivudine)
- Ziagen (abacavir)
- Trizivir (abacavir/lamivudine/zidovudine)
- Combivir (lamivudine/zidovudine)
- Epivir/3TC (lamivudine)
- Retrovir/AZT (zidovudine)
- Lexiva/Telzir (fosamprenavir)
- Viracept (nelfinavir)
- Rescriptor (delavirdine mesylate)
Cost
A year supply of Triumeq costs around $33,000 as it is still under patent so not available as a generic.[2]
In July 2015, ViiV Healthcare struck a deal with Shanghai-based Desano Pharmaceuticals for a cheaper supply of dolutegravir (Tivicay) with the goal of cutting the cost in China and other developing countries. After approval of Tivicay in 2013, it came with a retail cost of $14,000 per year in the United States.[13]
See also
References
- "FDA Approves Triumeq". drugs.com. August 22, 2014.
- "Login". online.lexi.com. Retrieved 2015-12-10.
- "A Trial Comparing GSK1349572 50mg Plus Abacavir/Lamivudine Once Daily to Atripla (Also Called The SINGLE Trial) - Study Results - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2015-12-15.
- "Triumeq | ViiV Healthcare". www.viivhealthcare.com. Retrieved 2015-12-15.
- FDA: Potential Risk of Neural Tube Birth Defects
- United States Patent: 8129385 - Substituted 5-hydroxy-3,4,6,9,9a, 10-hexanhydro-2h-1-oxa04a,8a-diaza-anthracene-6,10-dioness, retrieved 2015-12-10
- "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 2015-12-10.
- "Tivicay (dolutegravir) and Triumeq (abacavir/dolutegravir/lamivudine) product labeling was updated". U.S. Food & Drug Administration (FDA). Archived from the original on 2015-12-22. Retrieved 2015-12-15.
- "Safety Information - Ziagen (abacavir sulfate) Tablets and Oral Solution". www.fda.gov. Retrieved 2015-12-15.
- "Who we are | ViiV Healthcare". www.viivhealthcare.com. Retrieved 2015-12-15.
- "Annual Report 2014 | GSK". www.gsk.com. Archived from the original on 2016-01-13. Retrieved 2015-12-15.
- "Our medicines | ViiV Healthcare". www.viivhealthcare.com. Retrieved 2015-12-15.
- "GSK's ViiV turns to Chinese company for cheap supply of Tivicay API". FiercePharmaManufacturing. Retrieved 2015-12-15.