Maraviroc
Maraviroc, brand-named Selzentry and Celsentri, is an antiretroviral drug in the CCR5 receptor antagonist class used in the treatment of HIV infection. It is also classed as an entry inhibitor. It also appeared to reduce graft-versus-host disease in patients treated with allogeneic bone marrow transplantation for leukemia, in a phase 1/2 study.[4][5]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /məˈrævɪrɒk/ mə-RAV-i-rok Selzentry: /sɛlˈzɛntri/ sel-ZEN-tree |
| Trade names | Selzentry, Celsentri |
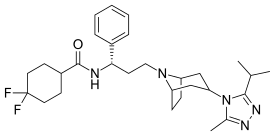
| Other names | 4,4-Difluoro-N-[(1S)-3-{(1R,3s,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl}-1-phenylpropyl] cyclohexanecarboxamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607076 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth (tablets, oral solution) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 23%[1] |
| Protein binding | ~76%[2] |
| Metabolism | Liver (CYP, predominantly CYP3A)[2] |
| Metabolites | Secondary amine formed by N-dealkylation (major) |
| Elimination half-life | 14–18 hours[2] (mean 16 hours)[3] |
| Excretion | Feces (76%), urine (20%)[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.124.927 |
| Chemical and physical data | |
| Formula | C29H41F2N5O |
| Molar mass | 513.666 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Medical uses
Two randomized, placebo-controlled clinical trials, known as MOTIVATE 1 & 2, compared 209 patients receiving optimized therapy plus a placebo to 426 patients receiving optimized therapy plus 150 mg maraviroc once daily and 414 patients receiving optimized therapy plus 150 mg maraviroc twice daily. At 48 weeks, 55% of participants receiving maraviroc once daily and 60% of participants receiving the drug twice daily achieved a viral load of less than 400 copies/mL compared with 26% of those taking placebo; about 44% of the once-daily and 45% of the twice-daily maraviroc group had a viral load of less than 50 copies/mL compared with about 23% of those who received placebo. In addition, those who received the entry inhibitor had a mean increase in CD4+ cells of 110 cells/μL in the once-daily group, 106 cells/μL in the twice-daily group, and 56 cells/μL in the placebo group.[6][7][8]
Side effects
Maraviroc can cause serious, life-threatening side effects. These include liver problems, skin reactions, and allergic reactions. An allergic reaction may happen before liver problems occur.[9] Official labeling of Selzentry has black box warning for hepatotoxicity.[2] The MOTIVATE trials showed no clinically relevant differences in safety between the maraviroc and placebo groups.[6]
Mechanism of action
Maraviroc is an entry inhibitor. Specifically, maraviroc is a negative allosteric modulator of the CCR5 receptor, which is found on the surface of certain human cells. The chemokine receptor CCR5 is an essential co-receptor for most HIV strains and necessary for the entry process of the virus into the host cell. The drug binds to CCR5, thereby blocking the HIV protein gp120 from associating with the receptor. HIV is then unable to enter human macrophages and T cells.[10] Because HIV can also use other coreceptors, such as CXCR4, an HIV tropism test such as a trofile assay must be performed to determine if the drug will be effective.[11]
Development and approval
Maraviroc, originally designated UK-427857, was developed by the drug company Pfizer in its UK labs located in Sandwich. On April 24, 2007 the U.S. Food and Drug Administration advisory panel reviewing maraviroc's New Drug Application unanimously recommended approval for the new drug,[12] and the drug received full FDA approval on August 6, 2007 for use in treatment experienced patients.[13]
On September 24, 2007, Pfizer announced that the European Commission approved maraviroc. Industry experts forecast annual maraviroc sales of $500 million by 2011.[14]
References
- Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK (April 2008). "Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects". British Journal of Clinical Pharmacology. 65 (Suppl 1): 60–7. doi:10.1111/j.1365-2125.2008.03137.x. PMC 2311408. PMID 18333867.
- "Selzentry (maraviroc) Tablets, for Oral Use; Oral Solution. Full Prescribing Information" (PDF). ViiV Healthcare, Research Triangle Park, NC 27709. Retrieved 20 November 2016.
- Abel S, Back DJ, Vourvahis M (2009). "Maraviroc: pharmacokinetics and drug interactions". Antiviral Therapy. 14 (5): 607–18. PMID 19704163.
- "HIV Drug Reduces Graft-versus-Host Disease in Bone Marrow Transplant Patients, Penn Study Shows – PR News". www.pennmedicine.org.
- Blocade of lymphocyte chemotaxis in visceral graft-versus-host disease, Ran Reshef et al., New England Journal of Medicine, 367:135 (July 12, 2012)
- Stephenson J (April 2007). "Researchers buoyed by novel HIV drugs: will expand drug arsenal against resistant virus". JAMA. 297 (14): 1535–6. doi:10.1001/jama.297.14.1535. PMID 17426263.
- Emmelkamp JM, Rockstroh JK (October 2007). "CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions--review of the literature". European Journal of Medical Research. 12 (9): 409–17. PMID 17933722.
- "Maraviroc reduces viral load in naive patients at 48 weeks". AIDS Patient Care and STDs. 21 (9): 703–4. September 2007. PMID 17941136.
- "Maraviroc (HIV treatment) Dosage, Side Effects". AIDSinfo.
- Levy JA (January 2009). "HIV pathogenesis: 25 years of progress and persistent challenges". AIDS. 23 (2): 147–60. doi:10.1097/QAD.0b013e3283217f9f. PMID 19098484.
- Biswas P, Tambussi G, Lazzarin A (May 2007). "Access denied? The status of co-receptor inhibition to counter HIV entry". Expert Opinion on Pharmacotherapy. 8 (7): 923–33. doi:10.1517/14656566.8.7.923. PMID 17472538.
- Gay News From 365Gay.com
- Krauskopf, Lewis (August 6, 2007). "Pfizer wins U.S. approval for new HIV drug". Reuters. Retrieved 2007-08-06.
- "Europe gives final approval to Pfizer HIV drug". 24 September 2007 – via www.reuters.com.
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 53" (PDF). World Health Organization. Retrieved 20 November 2016.
External links
- Selzentry (maraviroc) official web site
- BBC News story: Drug 'stops HIV's entry to cells'
- Maraviroc data at aidsmap
- Maraviroc early access program
- New HIV Drug Recommended for Approval
- Maraviroc at the US National Library of Medicine Medical Subject Headings (MeSH)
- U.S. National Center for Biotechnology Information: Medical Genetics Summaries - Maraviroc Therapy and CCR5 Genotype