Fostemsavir
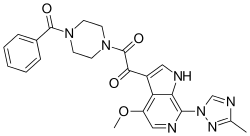
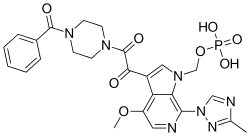
Fostemsavir (GSK3684934/BMS-663068) is an experimental HIV entry inhibitor and a prodrug of temsavir (BMS-626529). It is under development by ViiV Healthcare / GlaxoSmithKline for use in the treatment of HIV infection. By blocking the gp120 receptor of the virus, it prevents initial viral attachment to the host CD4+ T cell and entry into the host immune cell; its method of action is a first for HIV drugs.[1] Because it targets a different step of the viral lifecycle, it offers promise for individuals with virus that has become highly resistant to other HIV drugs.[2] Since gp120 is a highly conserved area of the virus, the drug is unlikely to promote resistance to itself.[3] Investigators found that enfuvirtide-resistant and ibalizumab-resistant HIV envelopes remained susceptible to Fostemsavir. Conversely, Fostemsavir-resistant HIV remained susceptible to all the entry inhibitors. Furthermore, HIV isolates that do not require the CD4 receptor for cell entry were also susceptible to Fostemsavir, and the virus did not escape the attachment inhibitor by becoming CD4-independent. Prior in vitro studies showed that Fostemsavir inhibits both CCR5-tropic and CXCR4-tropic HIV.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
{3-[(4-Benzoyl-1-piperazinyl)(oxo)acetyl]-4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-1-yl}methyl dihydrogen phosphate | |
| Other names
BMS-663068, GSK3684934 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C25H26N7O8P |
| Molar mass | 583.498 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- HIV Attachment Inhibitor BMS-663068 Looks Good in Early Studies
- HIV attachment inhibitor BMS-663068 shows good safety and efficacy in phase 2b study
- Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors
- HIV Attachment Inhibitor BMS-663068 Looks Good in Early Studies