Abacavir/lamivudine
Abacavir/lamivudine, sold under the brand name Kivexa among others, is a medication used to treat HIV/AIDS.[1] It is a fixed dose combination of abacavir and lamivudine.[1] It is generally recommended for use with other antiretrovirals.[1] It is commonly used as part of the preferred treatment in children.[2] It is taken by mouth as a tablet.[1]
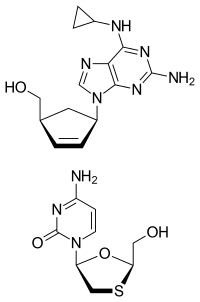 | |
| Combination of | |
|---|---|
| Abacavir | Nucleotide analogue reverse transcriptase inhibitor |
| Lamivudine | Nucleotide analogue reverse transcriptase inhibitor |
| Clinical data | |
| Trade names | Kivexa, Epzicom, others |
| MedlinePlus | a696011 |
| Pregnancy category |
|
| Routes of administration | by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| | |
Common side effects include trouble sleeping, headache, depression, feeling tired, nausea, rash, and fever.[1] Serious side effects may include high blood lactate levels, allergic reactions, and enlargement of the liver.[1] It is not recommended in people with a specific gene known as HLA-B*5701.[1] Safety in pregnancy has not been well studied but it appears to be okay.[3] Lamivudine and abacavir are both nucleoside reverse transcriptase inhibitors (NRTI).[1]
Abacavir/lamivudine was approved for medical use in the United States in 2004.[1] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[4] The wholesale cost in the developing world is about US$14.19 to $16.74 per month as of 2014.[5] As of 2015 the cost for a typical month of medication in the United States is more than $200.[6]
Society and culture
Names
It is marketed as Kivexa in most countries except for the United States, where it is branded as Epzicom.[7] It is marketed by ViiV Healthcare.
Legal challenges
Teva Pharmaceuticals and Lupin Ltd both filed abbreviated new drug applications (ANDAs) relating to the treatments of HIV using various combinations of abacavir, lamivudine and AZT, and challenging various patents. In 2013 the US District Court for the District of Delaware upheld the validity of a patent covering Epzicom and Tizivir. Other matters were subject to appeal or litigation as of 20 November 2014.[8]
See also
- Abacavir/lamivudine/zidovudine, tradename Trizivir
- Abacavir/dolutegravir/lamivudine, tradename Triumeq
References
- "Abacavir and Lamivudine Tablets". Teva Pharmaceuticals USA. Archived from the original on 6 February 2017. Retrieved 28 November 2016.
- The selection and use of essential medicines: Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children) (PDF). WHO. 2015. pp. 45–46. ISBN 9789240694941. Archived (PDF) from the original on 20 December 2016. Retrieved 8 December 2016.
- "Abacavir / lamivudine (Epzicom) Use During Pregnancy". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 4 December 2016.
- "World Health Organization model list of essential medicines: 21st list 2019". 2019. hdl:10665/325771. Cite journal requires
|journal=(help) - "Abacavir + Lamivudine". International Drug Price Indicator Guide. Retrieved 28 November 2016.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 59. ISBN 9781284057560.
- ViiV Healthcare: Kivexa Archived December 7, 2009, at the Wayback Machine
- "PROPOSED MAJOR TRANSACTION WITH NOVARTIS AG:Circular to Shareholders and Notice of General Meeting" (PDF). Glaxosmithkline. 20 November 2014. Archived from the original (PDF) on 2015-02-03. Retrieved 2015-02-03. Cite journal requires
|journal=(help)