NLP Workbench Web Services Safety Surveillance Domain Pilot Project
Two pilot projects will be completed to demonstrate how Natural Language Processing (NLP) Workbench Web Services can be used to meet specific requirements. They will focus on safety surveillance (described below) and cancer.
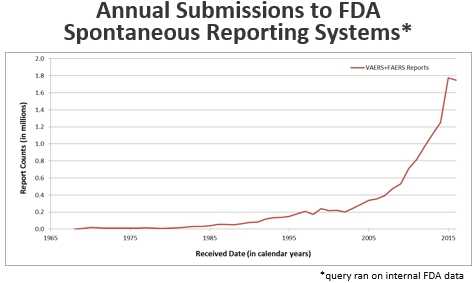
This chart shows that the number of annual submissions to FDA’s spontaneous reporting systems increased steadily from zero in 1968 to about 200,000 in 2002, then increased sharply to nearly 1.8 million in 2015.
Medical product administration may be associated with the onset of adverse events. An adverse event is any undesirable experience associated with the use of a medical product in a patient. Adverse event monitoring is—
- Easier in the pre-market phase with clinical trial data.
- Challenging in the post-market phase, with high volumes of data submitted to the U.S. Food and Drug Administration’s (FDA’s) spontaneous reporting systems (passive surveillance) and evaluated in FDA’s Sentinel Initiative (active surveillance).
FDA monitors post-market reports for medical products submitted annually to spontaneous reporting systems such as the Vaccine Adverse Event Reporting System (VAERS), which received about 54,000 reports in 2016, and the FDA Adverse Event Reporting System for drugs and biologics (FAERS), which received about 1.7 million reports in 2016.
Use Case 1: Extraction of Key Clinical Information from Safety Reports
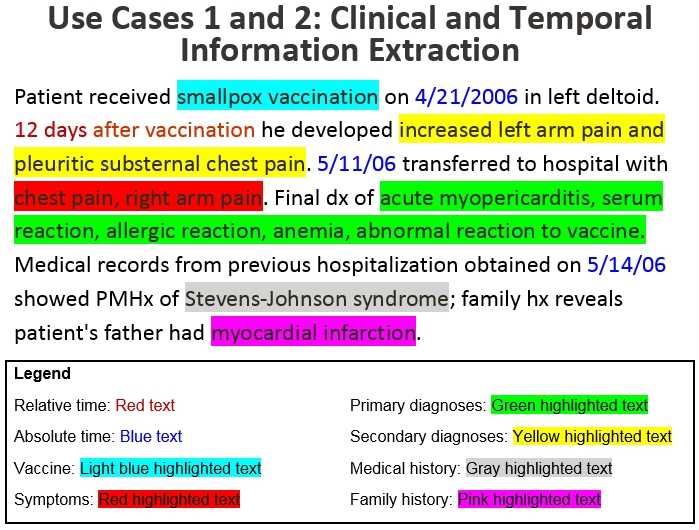
The text above illustrates the clinical and temporal information that will be extracted from a safety report using NLP. The narrative text is, “Patient received smallpox vaccination on 4/21/2006 in left deltoid. 12 days after vaccination he developed increased left arm pain and pleuritic substernal chest pain. 5/11/06 transferred to hospital with chest pain, right arm pain. Final dx of acute myopericarditis, serum reaction, allergic reaction, anemia, abnormal reaction to vaccine. Medical records from previous hospitalization obtained on 5/14/06 showed PMHx of Stevens-Johnson syndrome; family hx reveals patient’s father had myocardial infarction.”
- Relative time: “12 days after vaccination”
- Absolute time: “4/21/2006,” “5/11/06,” and “5/14/06”
- Vaccine: “Smallpox vaccination”
- Symptoms: “chest pain, right arm pain”
- Primary diagnoses: “acute myopericarditis, serum reaction, allergic reaction, anemia, abnormal reaction to vaccine”
- Secondary diagnoses: “increased left arm pain and pleuritic substernal chest pain”
- Medical history: “Stevens-Johnson syndrome”
- Family history: “myocardial infarction”
Use Case 2: Extraction of Temporal Information and Identification of Temporal Relationships in Safety Reports
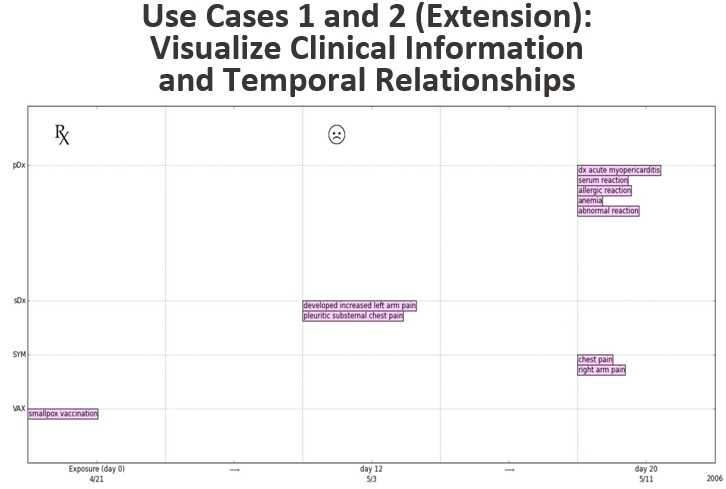
The chart above shows how the relationships between the temporal data will be identified and visualized after they are extracted from a safety report using NLP. The chart shows exposure to the smallpox vaccine on day 0 (4/21/2006). On day 12 (5/3/2006), symptoms develop. On day 20 (5/11/2006), the primary and secondary diagnoses are recorded.
Use Case 3: Summarization of Adverse Event Information
A summary is included in the safety narratives by combining the structured data fields (age, sex, and product names) with the clinical and temporal information from the safety narratives. Two types of summaries will be created for each safety report: a brief textual summary and a structured tabular summary.
- Page last reviewed: June 14, 2017
- Page last updated: June 14, 2017
- Content source:
- Maintained By:


 ShareCompartir
ShareCompartir