Lesson 1: Introduction to Epidemiology
Section 10: Chain of Infection
As described above, the traditional epidemiologic triad model holds that infectious diseases result from the interaction of agent, host, and environment. More specifically, transmission occurs when the agent leaves its reservoir or host through a portal of exit, is conveyed by some mode of transmission, and enters through an appropriate portal of entry to infect a susceptible host. This sequence is sometimes called the chain of infection.
Figure 1.19 Chain of Infection
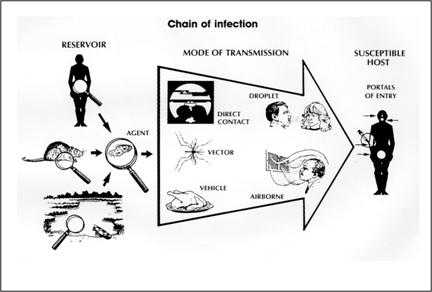
Source: Centers for Disease Control and Prevention. Principles of epidemiology, 2nd ed. Atlanta: U.S. Department of Health and Human Services;1992.
Reservoir
The reservoir of an infectious agent is the habitat in which the agent normally lives, grows, and multiplies. Reservoirs include humans, animals, and the environment. The reservoir may or may not be the source from which an agent is transferred to a host. For example, the reservoir of Clostridium botulinum is soil, but the source of most botulism infections is improperly canned food containing C. botulinum spores.
Human reservoirs. Many common infectious diseases have human reservoirs. Diseases that are transmitted from person to person without intermediaries include the sexually transmitted diseases, measles, mumps, streptococcal infection, and many respiratory pathogens. Because humans were the only reservoir for the smallpox virus, naturally occurring smallpox was eradicated after the last human case was identified and isolated.8
Human reservoirs may or may not show the effects of illness. As noted earlier, a carrier is a person with inapparent infection who is capable of transmitting the pathogen to others. Asymptomatic or passive or healthy carriers are those who never experience symptoms despite being infected. Incubatory carriers are those who can transmit the agent during the incubation period before clinical illness begins. Convalescent carriers are those who have recovered from their illness but remain capable of transmitting to others. Chronic carriers are those who continue to harbor a pathogen such as hepatitis B virus or Salmonella Typhi, the causative agent of typhoid fever, for months or even years after their initial infection. One notorious carrier is Mary Mallon, or Typhoid Mary, who was an asymptomatic chronic carrier of Salmonella Typhi. As a cook in New York City and New Jersey in the early 1900s, she unintentionally infected dozens of people until she was placed in isolation on an island in the East River, where she died 23 years later.(45)
Carriers commonly transmit disease because they do not realize they are infected, and consequently take no special precautions to prevent transmission. Symptomatic persons who are aware of their illness, on the other hand, may be less likely to transmit infection because they are either too sick to be out and about, take precautions to reduce transmission, or receive treatment that limits the disease.
Animal reservoirs. Humans are also subject to diseases that have animal reservoirs. Many of these diseases are transmitted from animal to animal, with humans as incidental hosts. The term zoonosis refers to an infectious disease that is transmissible under natural conditions from vertebrate animals to humans. Long recognized zoonotic diseases include brucellosis (cows and pigs), anthrax (sheep), plague (rodents), trichinellosis/trichinosis (swine), tularemia (rabbits), and rabies (bats, raccoons, dogs, and other mammals). Zoonoses newly emergent in North America include West Nile encephalitis (birds), and monkeypox (prairie dogs). Many newly recognized infectious diseases in humans, including HIV/AIDS, Ebola infection and SARS, are thought to have emerged from animal hosts, although those hosts have not yet been identified.
Environmental reservoirs. Plants, soil, and water in the environment are also reservoirs for some infectious agents. Many fungal agents, such as those that cause histoplasmosis, live and multiply in the soil. Outbreaks of Legionnaires disease are often traced to water supplies in cooling towers and evaporative condensers, reservoirs for the causative organism Legionella pneumophila.
Portal of exit
Portal of exit is the path by which a pathogen leaves its host. The portal of exit usually corresponds to the site where the pathogen is localized. For example, influenza viruses and Mycobacterium tuberculosis exit the respiratory tract, schistosomes through urine, cholera vibrios in feces, Sarcoptes scabiei in scabies skin lesions, and enterovirus 70, a cause of hemorrhagic conjunctivitis, in conjunctival secretions. Some bloodborne agents can exit by crossing the placenta from mother to fetus (rubella, syphilis, toxoplasmosis), while others exit through cuts or needles in the skin (hepatitis B) or blood-sucking arthropods (malaria).
Modes of transmission
An infectious agent may be transmitted from its natural reservoir to a susceptible host in different ways. There are different classifications for modes of transmission. Here is one classification:
- Direct
- Direct contact
- Droplet spread
- Indirect
- Airborne
- Vehicleborne
- Vectorborne (mechanical or biologic)
In direct transmission, an infectious agent is transferred from a reservoir to a susceptible host by direct contact or droplet spread.
Direct contact occurs through skin-to-skin contact, kissing, and sexual intercourse. Direct contact also refers to contact with soil or vegetation harboring infectious organisms. Thus, infectious mononucleosis (“kissing disease”) and gonorrhea are spread from person to person by direct contact. Hookworm is spread by direct contact with contaminated soil.
Droplet spread refers to spray with relatively large, short-range aerosols produced by sneezing, coughing, or even talking. Droplet spread is classified as direct because transmission is by direct spray over a few feet, before the droplets fall to the ground. Pertussis and meningococcal infection are examples of diseases transmitted from an infectious patient to a susceptible host by droplet spread.
Indirect transmission refers to the transfer of an infectious agent from a reservoir to a host by suspended air particles, inanimate objects (vehicles), or animate intermediaries (vectors).
Airborne transmission occurs when infectious agents are carried by dust or droplet nuclei suspended in air. Airborne dust includes material that has settled on surfaces and become resuspended by air currents as well as infectious particles blown from the soil by the wind. Droplet nuclei are dried residue of less than 5 microns in size. In contrast to droplets that fall to the ground within a few feet, droplet nuclei may remain suspended in the air for long periods of time and may be blown over great distances. Measles, for example, has occurred in children who came into a physician's office after a child with measles had left, because the measles virus remained suspended in the air.(46)
Vehicles that may indirectly transmit an infectious agent include food, water, biologic products (blood), and fomites (inanimate objects such as handkerchiefs, bedding, or surgical scalpels). A vehicle may passively carry a pathogen — as food or water may carry hepatitis A virus. Alternatively, the vehicle may provide an environment in which the agent grows, multiplies, or produces toxin — as improperly canned foods provide an environment that supports production of botulinum toxin by Clostridium botulinum.
Vectors such as mosquitoes, fleas, and ticks may carry an infectious agent through purely mechanical means or may support growth or changes in the agent. Examples of mechanical transmission are flies carrying Shigella on their appendages and fleas carrying Yersinia pestis, the causative agent of plague, in their gut. In contrast, in biologic transmission, the causative agent of malaria or guinea worm disease undergoes maturation in an intermediate host before it can be transmitted to humans (Figure 1.20).
Portal of entry
The portal of entry refers to the manner in which a pathogen enters a susceptible host. The portal of entry must provide access to tissues in which the pathogen can multiply or a toxin can act. Often, infectious agents use the same portal to enter a new host that they used to exit the source host. For example, influenza virus exits the respiratory tract of the source host and enters the respiratory tract of the new host. In contrast, many pathogens that cause gastroenteritis follow a so-called “fecal-oral” route because they exit the source host in feces, are carried on inadequately washed hands to a vehicle such as food, water, or utensil, and enter a new host through the mouth. Other portals of entry include the skin (hookworm), mucous membranes (syphilis), and blood (hepatitis B, human immunodeficiency virus).
Figure 1.20 Complex Life Cycle of Dracunculus medinensis (Guinea worm)
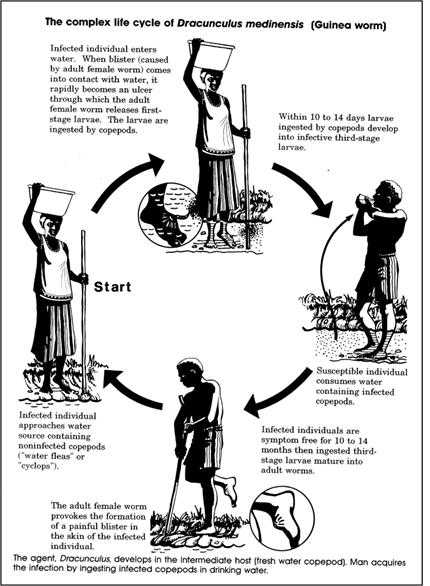
Source: Centers for Disease Control and Prevention. Principles of epidemiology, 2nd ed. Atlanta: U.S. Department of Health and Human Services;1992.
Host
The final link in the chain of infection is a susceptible host. Susceptibility of a host depends on genetic or constitutional factors, specific immunity, and nonspecific factors that affect an individual's ability to resist infection or to limit pathogenicity. An individual's genetic makeup may either increase or decrease susceptibility. For example, persons with sickle cell trait seem to be at least partially protected from a particular type of malaria. Specific immunity refers to protective antibodies that are directed against a specific agent. Such antibodies may develop in response to infection, vaccine, or toxoid (toxin that has been deactivated but retains its capacity to stimulate production of toxin antibodies) or may be acquired by transplacental transfer from mother to fetus or by injection of antitoxin or immune globulin. Nonspecific factors that defend against infection include the skin, mucous membranes, gastric acidity, cilia in the respiratory tract, the cough reflex, and nonspecific immune response. Factors that may increase susceptibility to infection by disrupting host defenses include malnutrition, alcoholism, and disease or therapy that impairs the nonspecific immune response.
Implications for public health
Knowledge of the portals of exit and entry and modes of transmission provides a basis for determining appropriate control measures. In general, control measures are usually directed against the segment in the infection chain that is most susceptible to intervention, unless practical issues dictate otherwise.
Interventions are directed at:
- Controlling or eliminating agent at source of transmission
- Protecting portals of entry
- Increasing host's defenses
For some diseases, the most appropriate intervention may be directed at controlling or eliminating the agent at its source. A patient sick with a communicable disease may be treated with antibiotics to eliminate the infection. An asymptomatic but infected person may be treated both to clear the infection and to reduce the risk of transmission to others. In the community, soil may be decontaminated or covered to prevent escape of the agent.
Some interventions are directed at the mode of transmission. Interruption of direct transmission may be accomplished by isolation of someone with infection, or counseling persons to avoid the specific type of contact associated with transmission. Vehicleborne transmission may be interrupted by elimination or decontamination of the vehicle. To prevent fecal-oral transmission, efforts often focus on rearranging the environment to reduce the risk of contamination in the future and on changing behaviors, such as promoting handwashing. For airborne diseases, strategies may be directed at modifying ventilation or air pressure, and filtering or treating the air. To interrupt vectorborne transmission, measures may be directed toward controlling the vector population, such as spraying to reduce the mosquito population.
Some strategies that protect portals of entry are simple and effective. For example, bed nets are used to protect sleeping persons from being bitten by mosquitoes that may transmit malaria. A dentist's mask and gloves are intended to protect the dentist from a patient's blood, secretions, and droplets, as well to protect the patient from the dentist. Wearing of long pants and sleeves and use of insect repellent are recommended to reduce the risk of Lyme disease and West Nile virus infection, which are transmitted by the bite of ticks and mosquitoes, respectively.
Some interventions aim to increase a host's defenses. Vaccinations promote development of specific antibodies that protect against infection. On the other hand, prophylactic use of antimalarial drugs, recommended for visitors to malaria-endemic areas, does not prevent exposure through mosquito bites, but does prevent infection from taking root.
Finally, some interventions attempt to prevent a pathogen from encountering a susceptible host. The concept of herd immunity suggests that if a high enough proportion of individuals in a population are resistant to an agent, then those few who are susceptible will be protected by the resistant majority, since the pathogen will be unlikely to “find” those few susceptible individuals. The degree of herd immunity necessary to prevent or interrupt an outbreak varies by disease. In theory, herd immunity means that not everyone in a community needs to be resistant (immune) to prevent disease spread and occurrence of an outbreak. In practice, herd immunity has not prevented outbreaks of measles and rubella in populations with immunization levels as high as 85% to 90%. One problem is that, in highly immunized populations, the relatively few susceptible persons are often clustered in subgroups defined by socioeconomic or cultural factors. If the pathogen is introduced into one of these subgroups, an outbreak may occur.
 Exercise 1.9
Exercise 1.9
Information about dengue fever is provided on the following pages. After studying this information, outline the chain of infection by identifying the reservoir(s), portal(s) of exit, mode(s) of transmission, portal(s) of entry, and factors in host susceptibility.
- Reservoirs:
- Portals of exit:
- Modes of transmission:
- Portals of entry:
- Factors in host susceptibility:
Dengue Fact Sheet
What is dengue?
Dengue is an acute infectious disease that comes in two forms: dengue and dengue hemorrhagic fever. The principal symptoms of dengue are high fever, severe headache, backache, joint pains, nausea and vomiting, eye pain, and rash. Generally, younger children have a milder illness than older children and adults.
Dengue hemorrhagic fever is a more severe form of dengue. It is characterized by a fever that lasts from 2 to 7 days, with general signs and symptoms that could occur with many other illnesses (e.g., nausea, vomiting, abdominal pain, and headache). This stage is followed by hemorrhagic manifestations, tendency to bruise easily or other types of skin hemorrhages, bleeding nose or gums, and possibly internal bleeding. The smallest blood vessels (capillaries) become excessively permeable (“leaky”), allowing the fluid component to escape from the blood vessels. This may lead to failure of the circulatory system and shock, followed by death, if circulatory failure is not corrected. Although the average case-fatality rate is about 5%, with good medical management, mortality can be less than 1%.
What causes dengue?
Dengue and dengue hemorrhagic fever are caused by any one of four closely related flaviviruses, designated DEN-1, DEN–2, DEN-3, or DEN-4.
How is dengue diagnosed?
Diagnosis of dengue infection requires laboratory confirmation, either by isolating the virus from serum within 5 days after onset of symptoms, or by detecting convalescent-phase specific antibodies obtained at least 6 days after onset of symptoms.
What is the treatment for dengue or dengue hemorrhagic fever?
There is no specific medication for treatment of a dengue infection. Persons who think they have dengue should use analgesics (pain relievers) with acetaminophen and avoid those containing aspirin. They should also rest, drink plenty of fluids, and consult a physician. Persons with dengue hemorrhagic fever can be effectively treated by fluid replacement therapy if an early clinical diagnosis is made, but hospitalization is often required.
How common is dengue and where is it found?
Dengue is endemic in many tropical countries in Asia and Latin America, most countries in Africa, and much of the Caribbean, including Puerto Rico. Cases have occurred sporadically in Texas. Epidemics occur periodically. Globally, an estimated 50 to 100 million cases of dengue and several hundred thousand cases of dengue hemorrhagic fever occur each year, depending on epidemic activity. Between 100 and 200 suspected cases are introduced into the United States each year by travelers.
How is dengue transmitted?
Dengue is transmitted to people by the bite of an Aedes mosquito that is infected with a dengue virus. The mosquito becomes infected with dengue virus when it bites a person who has dengue or DHF and after about a week can transmit the virus while biting a healthy person. Monkeys may serve as a reservoir in some parts of Asia and Africa. Dengue cannot be spread directly from person to person.
Who has an increased risk of being exposed to dengue?
Susceptibility to dengue is universal. Residents of or visitors to tropical urban areas and other areas where dengue is endemic are at highest risk of becoming infected. While a person who survives a bout of dengue caused by one serotype develops lifelong immunity to that serotype, there is no cross-protection against the three other serotypes.
What can be done to reduce the risk of acquiring dengue?
There is no vaccine for preventing dengue. The best preventive measure for residents living in areas infested with Aedes aegypti is to eliminate the places where the mosquito lays her eggs, primarily artificial containers that hold water.
Items that collect rainwater or are used to store water (for example, plastic containers, 55-gallon drums, buckets, or used automobile tires) should be covered or properly discarded. Pet and animal watering containers and vases with fresh flowers should be emptied and scoured at least once a week. This will eliminate the mosquito eggs and larvae and reduce the number of mosquitoes present in these areas.
For travelers to areas with dengue, as well as people living in areas with dengue, the risk of being bitten by mosquitoes indoors is reduced by utilization of air conditioning or windows and doors that are screened. Proper application of mosquito repellents containing 20% to 30% DEET as the active ingredient on exposed skin and clothing decreases the risk of being bitten by mosquitoes. The risk of dengue infection for international travelers appears to be small, unless an epidemic is in progress.
Can epidemics of dengue hemorrhagic fever be prevented?
The emphasis for dengue prevention is on sustainable, community-based, integrated mosquito control, with limited reliance on insecticides (chemical larvicides and adulticides). Preventing epidemic disease requires a coordinated community effort to increase awareness about dengue/DHF, how to recognize it, and how to control the mosquito that transmits it. Residents are responsible for keeping their yards and patios free of sites where mosquitoes can be produced.
Source: Centers for Disease Control and Prevention [Internet]. Dengue Fever. [updated 2005 Aug 22]. Available from http://www.cdc.gov/ncidod/dvbid/dengue/index.htm.
References (This Section)
- Leavitt JW. Typhoid Mary: captive to the public's health. Boston: Beacon Press; 1996.
- Remington PL, Hall WN, Davis IH, Herald A, Gunn RA. Airborne transmission of measles in a physician's office. JAMA 1985;253:1575–7.
Image Description
Figure 1.19
Description: The chain of infection has 3 main parts. A reservoir such as a human and an agent such as an amoeba. The mode of transmission can include direct contact, droplets, a vector such as a mosquito, a vehicle such as food, or the airborne route. The susceptible host has multiple portals of entry such as the mouth or a syringe. Return to text.
Figure 1.20
Description: The agent Dracunculus medinensis, develops in the intermediate host (fresh water copepod). Man acquires the infection by ingesting infected copepods in drinking water.
An infected individual enters the water. When a blister (caused by adult female worm) comes into contact with water, it rapidly becomes an ulcer through which the adult female worm releases first-stage larvae. The larvae are ingested by copepods.
Within 10 to 14 days larvae ingested by the copepods develop into infective third stage larvae. The susceptible individual consumes water containing infected copepods. Infected individuals are symptom free for 10 to 14 months then ingested third-stage larvae mature into adult worms.
The adult female worm provokes the formation of a painful blister in the skin of the infected individual. The infected individual approaches water source containing noninfected copepods ("water fleas" or "Cyclops"). Then the cycle starts over. Return to text.
- Page last reviewed: May 18, 2012
- Page last updated: May 18, 2012
- Content source:


 ShareCompartir
ShareCompartir