Dolutegravir
Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS.[3] It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure.[4] It is taken by mouth.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tivicay |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613043 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a[2] |
| Protein binding | ≥98.9% |
| Metabolism | UGT1A1 and CYP3A |
| Elimination half-life | ~14 hours |
| Excretion | Feces (53%) and urine (18.9%) |
| Identifiers | |
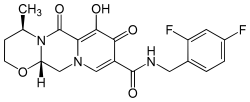
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.237.735 |
| Chemical and physical data | |
| Formula | C20H19F2N3O5 |
| Molar mass | 419.38 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include trouble sleeping, feeling tired, diarrhea, high blood sugar, and headache.[4] Severe side effects may include allergic reactions and liver problems.[4] There is tentative concerns that use during pregnancy can result in harm to the baby.[5] It is unclear if use during breastfeeding is safe.[4] Dolutegravir is an HIV integrase strand transfer inhibitor which blocks the functioning of HIV integrase which is needed for viral replication.[4]
Dolutegravir was approved for medical use in the United States in 2013.[4] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] In 2015, the cost of the medication in the United Kingdom was £499 per month.[3] Abacavir/dolutegravir/lamivudine, a combination with abacavir and lamivudine is also available.[4] As of 2019, the WHO recommends DTG as the first- and second-line treatment for all persons with HIV.[7]
Medical use
Dolutegravir is approved for use in a broad population of HIV-infected patients. It can be used to treat HIV-infected adults who have never taken HIV therapy (treatment-naïve) and HIV-infected adults who have previously taken HIV therapy (treatment-experienced), including those who have been treated with other integrase strand transfer inhibitors. Tivicay is also approved for children ages 12 years and older weighing at least 40 kilograms (kg) who are treatment-naïve or treatment-experienced but have not previously taken other integrase strand transfer inhibitors.[8]
Adverse effects
Common side effects of dolutegravir in clinical trials included insomnia and headache. Serious side effects included allergic reactions and abnormal liver function in patients who were also infected with hepatitis B or C.[9] The package insert warns against a mean rise in serum creatinine of 0.11 mg/dL due to inhibition of tubular secretion of creatinine and does not affect GFR.[2]
Pregnancy
There is tentative concerns that use during pregnancy can result in harm to the baby.[5] Effective birth control is thus recommended while on dolutegravir, with pregnancy testing before starting treatment.[10] Use during the first trimester should only occur if there is no alternative.[10]
History
In February, 2013 the Food and Drug Administration announced that it would fast track dolutegravir's approval process.[11] On August 13, 2013, dolutegravir was approved by the FDA. On November 4, 2013, dolutegravir was approved by Health Canada.[12] On January 16, 2014, it was approved by the European Commission for use throughout the European Union.[13]
In 2019 a triple combination therapy, with dolutegravir replacing efavirenz, was introduced as the first line treatment for all people with HIV by the South African Government (public) sector.
References
- "Dolutegravir (Tivicay) Use During Pregnancy". Drugs.com.
- "Tivicay® (dolutegravir) Tablets for Oral Use. Full Prescribing Information" (PDF). ViiV Healthcare, 2013. Archived from the original (PDF) on 3 January 2014. Retrieved 9 February 2014.
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 429. ISBN 9780857111562.
- "Dolutegravir Sodium". The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- "Dolutegravir Sodium Monograph for Professionals". Drugs.com. Retrieved 20 April 2019.
- "WHO Model List of Essential Medicines (20th List)" (PDF). World Health Organization. March 2017. Retrieved 29 June 2017.
- "WHO recommends dolutegravir as preferred HIV treatment option in all populations". www.who.int. Retrieved 2019-07-22.
- FDA approves new drug to treat HIV infection https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm364744.htm Aug. 12, 2013
- U.S. FDA approves GlaxoSmithKline's HIV drug Tivicay https://www.reuters.com/article/2013/08/12/us-glaxosmithkline-hivdrug-idUSBRE97B0WU20130812 Mon Aug 12, 2013 6:40pm EDT
- "Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 1-4 October 2018". European Medicines Agency. 5 October 2018.
- "GSK wins priority status for new HIV drug in U.S". Reuters. 16 February 2013. Retrieved 18 February 2013.
- "ViiV Healthcare receives approval for Tivicay™ (dolutegravir) in Canada for the treatment of HIV" (PDF). Archived from the original (PDF) on 12 November 2013. Retrieved 11 November 2013.
- EMA Tivicay information page: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002753/human_med_001720.jsp&mid=WC0b01ac058001d124