Pertussis
Printer friendly version [18 pages]
On this Page
Pertussis
- Acute infectious disease caused by Bordetella pertussis
- Outbreaks first described in 16th century
- Bordetella pertussis isolated in 1906
- Estimated 195,000 deaths worldwide in 2008
Pertussis, or whooping cough, is an acute infectious disease caused by the bacterium Bordetella pertussis. Outbreaks of pertussis were first described in the 16th century, and the organism was first isolated in 1906.
In the 20th century, pertussis was one of the most common childhood diseases and a major cause of childhood mortality in the United States. Before the availability of pertussis vaccine in the 1940s, more than 200,000 cases of pertussis were reported annually. Since widespread use of the vaccine began, incidence has decreased more than 80% compared with the prevaccine era.
Pertussis remains a major health problem among children in developing countries, with 195,000 deaths resulting from the disease in 2008 (World Health Organization estimate).
Bordetella pertussis
- Fastidious gram-negative bacteria
- Antigenic and biologically active components:
- pertussis toxin (PT)
- filamentous hemagglutinin (FHA)
- agglutinogens
- adenylate cyclase
- pertactin
- tracheal cytotoxin
Pertussis Pathogenesis
- Primarily a toxin-mediated disease
- Bacteria attach to cilia of respiratory epithelial cells
- Inflammation occurs which interferes with clearance of pulmonary secretions
- Pertussis antigens allow evasion of host defenses (lymphocytosis promoted but impaired chemotaxis)
Bordetella pertussis
B. pertussis is a small, aerobic gram-negative rod. It is fastidious and requires special media for isolation (see Laboratory Diagnosis).
B. pertussis produces multiple antigenic and biologically active products, including pertussis toxin (PT), filamentous hemagglutinin (FHA), agglutinogens, adenylate cyclase, pertactin, and tracheal cytotoxin. These products are responsible for the clinical features of pertussis disease, and an immune response to one or more produces immunity following infection. Immunity following B. pertussis infection does not appear to be permanent.
Pathogenesis
Pertussis is primarily a toxin-mediated disease. The bacteria attach to the cilia of the respiratory epithelial cells, produce toxins that paralyze the cilia, and cause inflammation of the respiratory tract, which interferes with the clearing of pulmonary secretions. Pertussis antigens appear to allow the organism to evade host defenses, in that lymphocytosis is promoted but chemotaxis is impaired. Until recently it was thought that B. pertussis did not invade the tissues. However, recent studies have shown the bacteria to be present in alveolar macrophages.
Clinical Features
Pertussis Clinical Features
- Incubation period 7-10 days (range 4-21 days)
- Insidious onset, similar to the common cold with nonspecific cough
- Fever usually minimal throughout course of illness
- Catarrhal stage
- 1-2 weeks
- Paroxysmal cough stage
- 1-6 weeks
- Convalescence
- weeks to months
The incubation period of pertussis is commonly 7–10 days, with a range of 4–21 days, and rarely may be as long as 42 days. The clinical course of the illness is divided into three stages.
The first stage, the catarrhal stage, is characterized by the insidious onset of coryza (runny nose), sneezing, low-grade fever, and a mild, occasional cough, similar to the common cold. The cough gradually becomes more severe, and after 1–2 weeks, the second, or paroxysmal stage, begins. Fever is generally minimal throughout the course of the illness.
It is during the paroxysmal stage that the diagnosis of pertussis is usually suspected. Characteristically, the patient has bursts, or paroxysms, of numerous, rapid coughs, apparently due to difficulty expelling thick mucus from the tracheobronchial tree. At the end of the paroxysm, a long inspiratory effort is usually accompanied by a characteristic high-pitched whoop. During such an attack, the patient may become cyanotic (turn blue). Children and young infants, especially, appear very ill and distressed. Vomiting and exhaustion commonly follow the episode. The person does not appear to be ill between attacks.
Paroxysmal attacks occur more frequently at night, with an average of 15 attacks per 24 hours. During the first 1 or 2 weeks of this stage, the attacks increase in frequency, remain at the same level for 2 to 3 weeks, and then gradually decrease. The paroxysmal stage usually lasts 1 to 6 weeks but may persist for up to 10 weeks. Infants younger than 6 months of age may not have the strength to have a whoop, but they do have paroxysms of coughing.
Pertussis Among Children, Adolescents and Adults
- Disease often milder than in infants and young children
- Infection may be asymptomatic, or may present as classic pertussis
- Persons with mild disease may transmit the infection
- Older persons often source of infection for children
In the convalescent stage, recovery is gradual. The cough becomes less paroxysmal and disappears in 2 to 3 weeks. However, paroxysms often recur with subsequent respiratory infections for many months after the onset of pertussis.
Adolescents, adults and children partially protected by the vaccine may become infected with B. pertussis but may have milder disease than infants and young children. Pertussis infection in these persons may be asymptomatic, or present as illness ranging from a mild cough illness to classic pertussis with persistent cough (i.e., lasting more than 7 days). Inspiratory whoop is not common.
Even though the disease may be milder in older persons, those who are infected may transmit the disease to other susceptible persons, including unimmunized or incompletely immunized infants. Older persons are often found to have the first case in a household with multiple pertussis cases, and are often the source of infection for children.
Pertussis Complications in Children
- Secondary bacterial pneumonia – most common
- Neurologic complications – seizures, encephalopathy more common among infants
- Otitis media
- Anorexia
- Dehydration
- Pneumothorax
- Epistaxis
- Subdural hematomas
- Hernias
- Rectal prolapse
Pertussis Complications in Adolescents and Adults
- Difficulty sleeping
- Urinary incontinence
- Pneumonia
- Rib fracture
Pertussis Laboratory Diagnosis
- Culture – gold standard
- Polymerase Chain Reaction (PCR)
- can confirm pertussis in an outbreak
- highly sensitive
- high false-positive rate
- Serology
- can confirm illness late in the course of infection
- many tests have unproven or unknown clinical accuracy
- Direct fluorescent antibody test
- low sensitivity
- variable specificity
- should not be used for laboratory confirmation
Complications
The most common complication, and the cause of most pertussis-related deaths, is secondary bacterial pneumonia. Young infants are at highest risk for acquiring pertussis-associated complications. Data from 1997–2000 indicate that pneumonia occurred in 5.2% of all reported pertussis cases, and among 11.8% of infants younger than 6 months of age.
Neurologic complications such as seizures and encephalopathy (a diffuse disorder of the brain) may occur as a result of hypoxia (reduction of oxygen supply) from coughing, or possibly from toxin. Neurologic complications of pertussis are more common among infants. Other less serious complications of pertussis include otitis media, anorexia, and dehydration. Complications resulting from pressure effects of severe paroxysms include pneumothorax, epistaxis, subdural hematomas, hernias, and rectal prolapse.
In 2008 through 2011 a total of 72 deaths from pertussis were reported to CDC. Children 3 months of age or younger accounted for 60 (83%) of these deaths. During 2008-2011, the annual mean of pertussis cases in infants was 3,132 (range 2,230 – 4,298), the mean of hospitalizations was 1,158 (range 687-1,459) and the mean of deaths was 16 (range 11-25).
Adolescents and adults may also develop complications of pertussis, such as difficulty sleeping, urinary incontinence, pneumonia, and rib fracture.
Laboratory Diagnosis
The diagnosis of pertussis is based on a characteristic clinical history (cough for more than 2 weeks with whoop, paroxysms, or posttussive vomiting) as well as a variety of laboratory tests (culture, polymerase chain reaction [PCR], and serology).
Culture is considered the gold standard laboratory test and is the most specific of the laboratory tests for pertussis. However, fastidious growth requirements make B. pertussis difficult to culture. The yield of culture can be affected by specimen collection, transportation, and isolation techniques. Specimens from the posterior nasopharynx, not the throat, should be obtained using Dacron® or calcium alginate (not cotton) swabs. Isolation rates are highest during the first 2 weeks of illness (catarrhal and early paroxysmal stages). Cultures are variably positive (30%–50%) and may take as long as 2 weeks, so results may be too late for clinical usefulness. Cultures are less likely to be positive if performed later in the course of illness (more than 2 weeks after cough onset) or on specimens from persons who have received antibiotics or have been vaccinated. Since adolescents and adults have often been coughing for several weeks before they seek medical attention, it is often too late for culture to be useful.
Polymerase chain reaction (PCR) is a rapid test and has excellent sensitivity. PCR tests vary in specificity, so obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time there is suspicion of a pertussis outbreak. Results should be interpreted along with the clinical symptoms and epidemiological information. PCR should be tested from nasopharyngeal specimens taken at 0-3 weeks following cough onset, but may provide accurate results for up to 4 weeks of cough in infants or unvaccinated persons. After the fourth week of cough, the amount of bacterial DNA rapidly diminishes, which increases the risk of obtaining falsely-negative results. PCR assay protocols that include multiple targets allow for speciation among Bordetella species. The high sensitivity of PCR increases the risk of false-positivity, but following some simple best practices can reduce the risk of obtaining inaccurate results.
Serologic testing could be useful for adults and adolescents who present late in the course of their illness, when both culture and PCR are likely to be negative. CDC and FDA have developed a serologic assay that has been extremely useful for confirming diagnosis, especially during suspected outbreaks. Many state public health labs have included this assay as part of their testing regimen for pertussis.Commercially, there are many different serologic tests used in United States with unproven or unknown clinical accuracy. CDC is actively engaged in better understanding the usefulness of these commercially available assays. Generally, serologic tests are more useful for diagnosis in later phases of the disease. For the CDC single point serology, the optimal timing for specimen collection is 2 to 8 weeks following cough onset, when the antibody titers are at their highest; however, serology may be performed on specimens collected up to 12 weeks following cough onset.
Because direct fluorescent antibody testing of nasopharyngeal secretions has been demonstrated in some studies to have low sensitivity and variable specificity, such testing should not be relied on as a criterion for laboratory confirmation.
An elevated white blood cell count with a lymphocytosis is usually present in classical disease of infants. The absolute lymphocyte count often reaches 20,000 or greater. However, there may be no lymphocytosis in some infants and children or in persons with mild or modified cases of pertussis. More information on the laboratory diagnosis of pertussis is available.
Medical Management
The medical management of pertussis cases is primarily supportive, although antibiotics are of some value. This therapy eradicates the organism from secretions, thereby decreasing communicability and, if initiated early, may modify the course of the illness. Recommended antibiotics are azithromycin, clarithromycin, and erythromycin. Trimethoprim-sulfamethoxasole can also be used.
Pertussis Epidemiology
- Reservoir
- Human Adolescents and adults
- Transmission
- respiratory droplets
- Communicability
- Maximum in catarrhal stage
- Secondary attack rate up to 80%
Pertussis – United States, 1940-2012
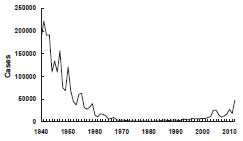
Source: National Notifiable Diseases Surveillance System, CDC
Pertussis – United States, 1980-2012
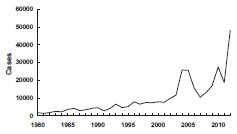
Source: National Notifiable Diseases Surveillance System, CDC
An antibiotic effective against pertussis should be administered to all close contacts of persons with pertussis, regardless of age and vaccination status. Revised treatment and postexposure prophylaxis recommendations were published in December 2005 (see reference list). All close contacts younger than 7 years of age who have not completed the four-dose primary series should complete the series with the minimal intervals. (see table in Appendix A [2 pages]). Close contacts who are 4–6 years of age and who have not yet received the second booster dose (usually the fifth dose of DTaP) should be vaccinated. The administration of Tdap to persons who have been exposed to a person with pertussis is not contraindicated, but the efficacy of postexposure use of Tdap is unknown.
Epidemiology
Occurrence
Pertussis occurs worldwide.
Reservoir
Pertussis is a human disease. No animal or insect source or vector is known to exist. Adolescents and adults are an important reservoir for B. pertussis and are often the source of infection for infants.
Transmission
Transmission most commonly occurs by the respiratory route through contact with respiratory droplets, or by contact with airborne droplets of respiratory secretions. Transmission occurs less frequently by contact with freshly contaminated articles of an infected person.
Temporal Pattern
Pertussis has no distinct seasonal pattern, but it may increase in the summer and fall.
Communicability
Pertussis is highly communicable, as evidenced by secondary attack rates of 80% among susceptible household contacts. Persons with pertussis are most infectious during the catarrhal period and the first 2 weeks after cough onset (i.e., approximately 21 days).
Secular Trends in the United States
Before the availability of vaccine, pertussis was a common cause of morbidity and mortality among children. During the 6-year period from 1940 through 1945, more than 1 million cases of pertussis were reported, an average of 175,000 cases per year (incidence of approximately 150 cases per 100,000 population).
Following introduction of whole-cell pertussis vaccine in the 1940s, pertussis incidence gradually declined, reaching 15,000 reported cases in 1960 (approximately 8 per 100,000 population). By 1970, annual incidence was fewer than 5,000 cases per year, and during 1980–1990, an average of 2,900 cases per year were reported (approximately 1 per 100,000 population).
Pertussis incidence has been gradually increasing since the early 1980s. A total of 25,827 cases was reported in 2004, the largest number since 1959. The reasons for the increase are not clear. A total of 27,550 pertussis cases and 27 pertussis-related deaths were reported in 2010. Case counts for 2012 have surpassed 2010, with 48,277 pertussis cases, with 13 deaths in infants (provisional).
During 2001–2003, the highest average annual pertussis incidence was among infants younger than 1 year of age (55.2 cases per 100,000 population), and particularly among children younger than 6 months of age (98.2 per 100,000 population). In 2002, 24% of all reported cases were in this age group. However, in recent years, adolescents (11–18 years of age) and adults (19 years and older) have accounted for an increasing proportion of cases. During 2001–2003, the annual incidence of pertussis among persons aged 10–19 years increased from 5.5 per 100,000 in 2001, to 6.7 in 2002, and 10.9 in 2003. In 2004 and 2005, approximately 60% of reported cases were among persons 11 years of age and older. Increased recognition and diagnosis of pertussis in older age groups probably contributed to this increase of reported cases among adolescents and adults. In 2010, the United States experienced another peak in cases with approximately 27,000 cases and the emergence of disease in children 7-10 years of age. In 2012, case counts continued to be elevated among children 7-10 years; however, reports of disease were also elevated among adolescents aged 13 and 14, which has not been observed since the introduction of Tdap. The epidemiology of pertussis has changed in recent years, with an increasing burden of disease among fully-vaccinated children and adolescents, which is likely being driven by the transition to acellular vaccines in the 1990s.
Pertussis Surveillance
See information about pertussis surveillance and information about the case definition, and case classification.
Whole-Cell Pertussis Vaccine
- Developed in mid-1930s and combined as DTP in mid-1940s
- DTP – 70%-90% effective after 4 dose
- Little to no protection after 5-10 years
- Local adverse reactions common
Pertussis Vaccine
Whole-Cell Pertussis Vaccine
Whole-cell pertussis vaccine is composed of a suspension of formalin-inactivated B. pertussis cells. Whole-cell pertussis vaccines were first licensed in the United States in 1914 and became available combined with diphtheria and tetanus toxoids (as DTP) in 1948.
Based on controlled efficacy trials conducted in the 1940s and on subsequent observational efficacy studies, a primary series of four doses of whole-cell DTP vaccine was 70%–90% effective in preventing serious pertussis disease. Protection decreased with time, resulting in little or no protection 5 to 10 years following the last dose. Local reactions such as redness, swelling, and pain at the injection site occurred following up to half of doses of whole-cell DTP vaccines. Fever and other mild systemic events were also common. Concerns about safety led to the development of more purified (acellular) pertussis vaccines that are associated with a lower frequency of adverse reactions. Whole-cell pertussis vaccines are no longer available in the United States but are still used in many other countries.
Acellular Pertussis Vaccine
Characteristics
Pertussis-containing Vaccines
- DTaP (pediatric)
- approved for children 6 weeks through 6 years (to age 7 years)
- Tdap (adolescent and adult)
- approved for persons 10 years and older (Boostrix) and 10 through 64 years (Adacel)
-
Composition* of Acellular Pertussis Vaccines
Product PT FHA PERT FIM Infanrix 25 25 8 — Daptacel 10 5 3 5 Boostrix 8 8 2.5 — Adacel 2.5 5 3 5 *mcg per dose
Acellular pertussis vaccines are subunit vaccines that contain purified, inactivated components of B. pertussis cells. Several acellular pertussis vaccines have been developed for different age groups; these contain different pertussis components in varying concentrations. Acellular pertussis vaccines are available only as combinations with tetanus and diphtheria toxoids.
Pediatric Formulation (DTaP)
Two pediatric acellular pertussis vaccines are currently available for use in the United States. Both vaccines are combined with diphtheria and tetanus toxoids as DTaP and are approved for children 6 weeks through 6 years of age (to age 7 years). Infanrix (GlaxoSmithKline) contains three antigens, mostly pertussis toxin (PT) and FHA. Daptacel (sanofi pasteur) contains five components, PT, FHA, pertactin, and fimbriae types 2 and 3. Neither of the available DTaP vaccines contains thimerosal as a preservative. Infanrix is supplied in single-dose vials or syringes, and Daptacel is supplied in single-dose vials only.
Adolescent and Adult Formulation (Tdap)
Acellular pertussis–containing vaccines were first licensed for adolescents and adults in 2005. Two vaccines are currently available. Both vaccines are combined with tetanus toxoid and a reduced amount of diphtheria toxoid compared with pediatric DTaP (that is, similar quantities of tetanus and diphtheria toxoid to adult formulation Td). Boostrix (GlaxoSmithKline) is approved for persons 10 years of age and older, and contains three pertussis antigens (PT, FHA, and pertactin) in a reduced quantity compared with the GlaxoSmithKline pediatric formulation. The vaccine contains aluminum hydroxide as an adjuvant and does not contain a preservative. Adacel (sanofi pasteur) is approved for persons 10 through 64 years of age. It contains the same five pertussis components as Daptacel but with a reduced quantity of PT. Adacel contains aluminum phosphate as an adjuvant and does not contain a preservative. Both vaccines are supplied in single-dose vials or syringes.
Immunogenicity and Vaccine Efficacy
DTaP
Since 1991, several studies conducted in Europe and Africa have evaluated the efficacy of DTaP vaccines administered to infants. These studies varied in type and number of vaccines, design, case definition, and laboratory method used to confirm the diagnosis of pertussis, so comparison among studies must be made with caution. Point estimates of vaccine efficacy ranged from 80% to 85% for vaccines currently licensed in the United States. Confidence intervals for vaccine efficacy overlap, suggesting that none of the vaccines is significantly more effective than the others. When studied, the acellular pertussis vaccine was significantly more effective than whole-cell DTP. Mild local and systemic adverse reactions and more serious adverse reactions (such as high fever, persistent crying, hypotonic-hyporesponsive episodes, and seizures) occurred less frequently among infants vaccinated with acellular pertussis vaccines than among those vaccinated with whole-cell DTP.
Routine DTaP Primary Vaccination Schedule
| Dose | Age | Minimum Interval |
|---|---|---|
| Primary 1 | 6 weeks – 2 months | — |
| Primary 2 | 4 months | 4 weeks |
| Primary 3 | 6 months | 4 weeks |
| Primary 4 | 15-18 months | 6 months |
DTaP Fourth Dose
- Recommended at 15-18 months*
- May be given at 12 months of age if:
- 6 months since DTaP3, and
- unlikely to return at 15-18 months
*15-20 months for Daptacel
School Entry (Fifth) Dose
- Fifth dose recommended when 4th dose given before age 4 years
- All DTaP vaccines are licensed for 5th dose after DTaP series
Interchangeability of Different Brands of DTaP Vaccine
- Series should be completed with same brand of vaccine if possible
- Limited data suggest that “mix and match” DTaP schedules do not adversely affect safety and immunogenicity
- Use different brand of DTaP if necessary
Tdap Vaccines
- Boostrix (GlaxoSmithKline)
- approved for persons 10 years of age and older
- Adacel (sanofi pasteur)
- approved for persons 11 through 64 years of age
Tdap
Adolescent and adult formulation Tdap vaccines were licensed on the basis of noninferiority of the serologic response to the various components compared with each company’s pediatric DTaP formulation (Infanrix and Daptacel) among persons who had received pediatric DTaP or DTP in childhood. For both vaccines, the antibody response to a single dose of Tdap was similar to that following three doses of DTaP in infants. This type of study is known as “bridging.” The new vaccines are assumed to have similar clinical efficacy as DTaP vaccine since a similar level of antibody to the components was achieved.
Vaccination Schedule and Use
DTaP
The series of DTaP vaccine consists of five doses, the first three doses given at 4- to 8-week intervals (minimum of 4 weeks), beginning at 6 weeks to 2 months of age. The fourth dose is given 6–12 months after the third to maintain adequate immunity for the ensuing preschool years. DTaP should be administered simultaneously with all other indicated vaccines.
The fourth dose of all brands of DTaP is licensed, and recommended by ACIP, to be administered at 15–18 months of age (15–20 months for Daptacel). However, ACIP recommends that in certain circumstances the fourth dose be given earlier than 15 months of age. The fourth dose of DTaP may be given if the child is at least 12 months of age, and at least 6 months have elapsed since the third dose of pertussis vaccine was given, and, in the opinion of the immunization provider, the child is unlikely to return for an additional visit at 15–18 months of age. All three of these criteria should be met in order to administer the fourth dose of DTaP at 12–14 months of age.
Children who received four doses before the fourth birthday should receive a fifth dose of DTaP before entering school. This booster dose is not necessary (but may be given) if the fourth dose in the series was given on or after the fourth birthday. The fifth dose increases antibody levels and may decrease the risk of school-age children transmitting the disease to younger siblings who are not fully vaccinated.
ACIP recommends that the series be completed with the same brand of DTaP vaccine if possible. However, limited data suggest that “mix and match” DTaP schedules do not adversely affect safety and immunogenicity. If the vaccine provider does not know or have available the type of DTaP vaccine previously administered to a child, any available DTaP vaccine should be used to continue or complete the vaccination series. Unavailability of the vaccine used for earlier doses is not a reason for missing the opportunity to administer a dose of acellular pertussis vaccine for which the child is eligible.
Interruption of the recommended schedule or delayed doses does not lead to a reduction in the level of immunity reached on completion of the primary series. There is no need to restart a series regardless of the time that has elapsed between doses.
Tdap
Tdap Recommendations
- A single dose of Tdap is recommended for
- adolescents 11 through 18 years of age
- adults 19 and older
- children 7-10 years of age who are not fully vaccinated against pertussis*
* “Not fully vaccinated” against pertussis is defined as having received fewer than 4 doses of DTaP, or having received 4 doses of DTaP but the last dose was prior to age 4 years. See MMWR 2011;60(No.1):13-5.
Both Tdap vaccines are approved by the Food and Drug Administration for a single (booster) dose for persons who have completed the recommended childhood DTP/DTaP vaccination series. Boostrix is approved for persons 10 years of age and older; Adacel is approved for persons 10 through 64 years of age.
ACIP recommends a single Tdap dose for persons aged 11 through 18 years who have completed the recommended childhood diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis (DTP/DTaP) vaccination series and for adults aged 19 through 64 years.
Children 7 through 10 years of age who are not fully vaccinated against pertussis (defined as having received fewer than 4 doses of DTaP, or having received 4 doses of DTaP but the last dose was prior to age 4 years) and who do not have a contraindication to pertussis vaccine should receive a single dose of Tdap to provide protection against pertussis. If additional doses of tetanus and diphtheria toxoid- containing vaccines are needed, then children 7 through 10 years of age should be vaccinated according to the catch-up schedule, with Tdap preferred as the first dose. Either brand of Tdap may be used. Currently, Tdap is recommended only for a single dose across all age groups.
Tdap Recommendations for Pregnant Women
- Providers of prenatal care should implement a Tdap vaccination program for pregnant women who previously have not received Tdap
- Administer Tdap in each pregnancy, preferably at 27 through 36 weeks gestation
- If not administered during pregnancy, Tdap should be administered immediately postpartum, for women not previously vaccinated with Tdap
MMWR 2013;62(No. 7):131-5
Adults 19 years of age and older who previously have not received Tdap should receive a single dose of Tdap to protect against pertussis and reduce the likelihood of transmission. For adults 19-64 years of age either brand of Tdap may be used. Adults 65 years or older should be vaccinated with Boostrix if feasible. However, either vaccine administered to a person 65 years or older is immunogenic and would provide protection. A dose of either vaccine would be considered valid.
Tdap can be administered regardless of interval since the last tetanus- or diphtheria-toxoid containing vaccine. After receipt of Tdap, persons should continue to receive Td for routine booster immunization against tetanus and diphtheria, generally every 10 years.
ACIP recommends that providers of prenatal care implement a Tdap immunization program for all pregnant women. Healthcare personnel should administer a dose of Tdap during each pregnancy, irrespective of the patient’s prior history of receiving Tdap. To maximize the maternal antibody response and passive antibody transfer to the infant, optimal timing for Tdap administration is between 27 and 36 weeks gestation although Tdap may be given at any time during pregnancy. For women not previously vaccinated with Tdap, if Tdap is not administered during pregnancy, Tdap should be administered immediately postpartum. No study has assessed the safety of repeated doses of Tdap in pregnant women. CDC will monitor and assess the safety of Tdap use during pregnancy.
Tdap Vaccine and Healthcare Personnel
- Healthcare personnel should receive a single dose of Tdap as soon as feasible*
- Priority should be given to vaccination of healthcare personnel who have direct contact with infants 12 months of age and younger
*if they have not previously received Tdap.
MMWR 2006;55(RR-17):1-37
Tdap For Persons Without A History of DTP or DTaP
- All adolescents and adults should have documentation of having received a series of DTaP, DTP, DT, or Td
- Persons without documentation should receive a series of 3 vaccinations
- One dose should be Tdap, preferably the first
Studies on the persistence of antipertussis antibodies following a dose of Tdap show antibody levels in healthy, nonpregnant adults peak during the first month after vaccination, with antibody levels declining after 1 year. The decline in antibody levels in pregnant women likely would be similar. Because antibody levels wane substantially during the first year after vaccination, ACIP concluded a single dose of Tdap at one pregnancy would be insufficient to provide protection for subsequent pregnancies.
ACIP also recommends that adolescents and adults (e.g., parents, siblings, grandparents, childcare providers, and healthcare personnel) who have or anticipate having close contact with an infant younger than 12 months of age should receive a single dose of Tdap to protect against pertussis if they have not previously received Tdap. Ideally, these persons should receive Tdap at least 2 weeks before beginning close contact with the infant.
Healthcare personnel should receive a single dose of Tdap as soon as feasible if they have not previously received Tdap and regardless of the time since their most recent Td vaccination. Priority should be given to vaccination of healthcare personnel who have direct contact with infants 12 months of age and younger.
Tdap vaccine may be given at the same visit, or any time before or after any other vaccine.
Immunity following pertussis is not permanent. Persons with a history of pertussis should receive a single dose of Tdap if it is otherwise indicated.
All adolescents and adults should have documentation of having received a primary series of at least three doses of tetanus and diphtheria toxoids during their lifetime. A person without such documentation should receive a series of three doses of tetanus- and diphtheria-containing vaccine. One of these doses, preferably the first, should be Tdap. The remaining two doses should be adult formulation Td.
Combination Vaccines Containing DTaP
Pediarix
- DTaP – Hep B – IPV combination
- Minimum age 6 weeks
- Approved for 3 doses at 2, 4 and 6 months
- Not approved for 4th or 5th booster dose of DTaP or IPV series
- Licensed for children 6 weeks through 6 years of age
- May be used interchangeably with other pertussis-containing vaccines if necessary
- Can be given at 2, 4, and 6 months in infants who received a birth dose of hepatitis B vaccine (total of 4 doses)
- May be used in infants whose mothers are HBsAg positive or status is not known*
*Off-label ACIP recommendation [8 pages]
Pentacel Vaccine
- Contains lyophilized Hib (ActHIB) vaccine that is reconstituted with a liquid DTaP-IPV solution
- Approved for doses 1 through 4 among children 6 weeks through 4 years of age
- The DTaP-IPV solution should not be used separately (i.e., only use to reconstitute the Hib component)
Pediarix
In 2002, the FDA approved Pediarix (GlaxoSmithKline), the first pentavalent (5 component) combination vaccine licensed in the United States. Pediarix contains DTaP (Infanrix), hepatitis B (Engerix-B), and inactivated polio vaccines.
The minimum age for the first dose of Pediarix is 6 weeks, so it cannot be used for the birth dose of the hepatitis B series. Pediarix is approved for the first three doses of the DTaP and inactivated polio vaccine (IPV) series, which are usually given at about 2, 4, and 6 months of age; it is not approved for fourth or fifth (booster) doses of the DTaP or IPV series. However, Pediarix is approved for use through 6 years of age. A child who is behind schedule can receive Pediarix as long as it is given for doses 1, 2, or 3 of the series, and the child is younger than 7 years of age.
A dose of Pediarix inadvertently administered as the fourth or fifth dose of the DTaP or IPV series does not need to be repeated.
Pediarix may be used interchangeably with other pertussis-containing vaccines if necessary (although ACIP prefers the use of the same brand of DTaP for all doses of the series, if possible). It can be given at 2, 4, and 6 months to infants who received a birth dose of hepatitis B vaccine (total of four doses of hepatitis B vaccine). Although not labeled for this indication by FDA, Pediarix may be used in infants whose mothers are HBsAg positive or whose HBsAg status is not known.
Pentacel
Pentacel is a combination vaccine that contains lyophilized Hib (ActHIB) vaccine that is reconstituted with a liquid DTaP-IPV solution. The vaccine was licensed by FDA in June 2008. Pentacel is licensed by FDA for doses 1 through 4 of the DTaP series among children 6 weeks through 4 years of age. The minimum intervals for Pentacel are determined by the DTaP component. The first three doses must be separated by at least 4 weeks. The fourth dose must be separated from the third by at least 6 calendar months, and not administered before 12 months of age. Pentacel should not be used for the fifth dose of the DTaP series, or for children 5 years or older regardless of the number of prior doses of the component vaccines.
The DTaP-IPV solution is licensed only for use as the diluent for the lyophilized Hib component and should not be used separately.
Kinrix
Kinrix is a combination vaccine that contains DTaP and inactivated poliovirus vaccine (IPV) that is produced by GlaxoSmithKline. It was approved by the FDA in 2008. Kinrix is licensed only for the fifth dose of DTaP and fourth dose of IPV in children 4 through 6 years of age whose previous DTaP vaccine doses have been with Infanrix and/or Pediarix for the first three doses and Infanrix for the fourth dose. However, if Kinrix is administered to children who received another brand of DTaP for prior DTaP doses the Kinrix dose does not need to be repeated.
Pertussis Vaccine Use in Children with Underlying Neurologic Disorders
| Underlying Condition | Recommendation |
|---|---|
| Prior seizure | Delay and assess* |
| Suspected neurologic disorder | Delay and assess* |
| Neurologic event between doses | Delay and assess* |
| Stable/resolved neurologic condition | Vaccinate |
*vaccinate after treatment initiated and condition stabilized
Other DTaP Issues
In certain circumstances, vaccination with DTaP vaccine should be delayed until a child with a known or suspected neurologic condition has been evaluated, treatment initiated, and the condition stabilized. These conditions include the presence of an evolving neurologic disorder (e.g., uncontrolled epilepsy, infantile spasms, and progressive encephalopathy), a history of seizures that has not been evaluated, or a neurologic event that occurs between doses of pertussis vaccine.
A family history of seizures or other neurologic diseases, or stable or resolved neurologic conditions (e.g., controlled idiopathic epilepsy, cerebral palsy, developmental delay) are not contraindications to pertussis vaccination.
Reducing the dose of DTaP vaccine or giving the full dose in multiple smaller doses may result in an altered immune response and inadequate protection. Furthermore, there is no evidence that the chance of a significant vaccine reaction is likely to be reduced by this practice. The use of multiple reduced doses that together equal a full immunizing dose, or the use of smaller, divided doses is not endorsed or recommended. Any vaccination using less than the standard dose should not be counted, and the person should be revaccinated according to age.
Because immunity from pertussis disease wanes, children who have recovered from documented pertussis should be vaccinated with pertussis vaccines according to the routine schedules.
Contraindications and Precautions to Vaccination
DTaP Contraindications
- Severe allergic reaction to vaccine component or following a prior dose
- Encephalopathy not due to another identifiable cause occurring within 7 days after vaccination
DTaP Precautions*
- Moderate or severe acute illness
- Temperature 105°F (40.5°C) or higher within 48 hours with no other identifiable cause
- Collapse or shock-like state (hypotonic-hyporesponsive episode) within 48 hours
- Persistent, inconsolable crying lasting 3 hours or longer, occurring within 48 hours
- Convulsions with or without fever occurring within 3 days
*may consider use in outbreaks
Tdap Contraindications
- Severe allergic reaction to vaccine component or following a prior dose
- Encephalopathy not due to another identifiable cause occurring within 7 days after vaccination with a pertussis-containing vaccine
DTaP
Contraindications to further vaccination with DTaP are a severe allergic reaction (anaphylaxis) to a vaccine component or following prior dose of vaccine, and encephalopathy not due to another identifiable cause occurring within 7 days after vaccination.
Moderate or severe acute illness is a precaution to vaccination. Children with mild illness, such as otitis media or upper respiratory infection, should be vaccinated. Children for whom vaccination is deferred because of moderate or severe acute illness should be vaccinated when their condition improves.
Certain infrequent adverse reactions following DTaP vaccination are considered to be precautions for subsequent doses of pediatric pertussis vaccine. These adverse reactions are a temperature of 105°F (40.5°C) or higher within 48 hours that is not due to another identifiable cause; collapse or shock-like state (hypotonic-hyporesponsive episode) within 48 hours; persistent, inconsolable crying lasting 3 hours or longer, occurring within 48 hours; and convulsions with or without fever occurring within 3 days.
There are circumstances (e.g., during a communitywide outbreak of pertussis) in which the benefit of vaccination outweighs the risk, even if one of the four precautionary adverse reactions occurred following a prior dose. In these circumstances, one or more additional doses of pertussis vaccine should be considered. DTaP should be used in these circumstances.
Tdap
Tdap is contraindicated for persons with a history of a severe allergic reaction to a vaccine component or following a prior dose of vaccine. Tdap is also contraindicated for persons with a history of encephalopathy not due to another identifiable cause occurring within 7 days after administration of a pertussis-containing vaccine.
Precautions to Tdap include a history of Guillain-Barré syndrome within 6 weeks after a previous dose of tetanus toxoid-containing vaccine and a progressive neurologic disorder (such as uncontrolled epilepsy or progressive encephalopathy) until the condition has stabilized. Persons with a history of a severe local reaction (Arthus reaction) following a prior dose of a tetanus and/or diphtheria toxoid-containing vaccine should generally not receive Tdap or Td vaccination until at least 10 years have elapsed after the last Td-containing vaccine. Moderate or severe acute illness is a precaution to vaccination. Persons for whom vaccination is deferred because of moderate or severe acute illness should be vaccinated when their condition improves.
Tdap Precautions
- History of Guillain-Barré syndrome within 6 weeks after a prior dose of tetanus toxoid-containing vaccine
- Progressive neurologic disorder until the condition has stabilized
- History of a severe local reaction (Arthus reaction) following a prior dose of a tetanus and/or diphtheria toxoid-containing vaccine
- Moderate or severe acute illness
DTap Adverse Reactions
- Local reactions (pain, redness, swelling)
- 20%-40%
- Temp of 101°F
- 3%-5%
- More severe adverse reactions
- not common
- Local reactions more common following 4th and 5th doses
Adverse Reactions Following the 4th and 5th DTaP Dose
- Local adverse reactions and fever increased with 4th and 5th doses of DTaP
- Reports of swelling of entire limb
- Extensive swelling after 4th dose NOT a contraindication to 5th dose
As noted above, certain conditions following DTaP vaccine, such as temperature of 105°F or higher, collapse or shock-like state, persistent crying, or convulsions with or without fever are a precaution to subsequent doses of DTaP. However, occurrence of one of these adverse reactions following DTaP vaccine in childhood is not a contraindication or precaution to administration of Tdap to an adolescent or adult. A history of extensive limb swelling following DTaP is not a contraindication to Tdap vaccination. A stable neurologic disorder (such as controlled seizures or cerebral palsy), breastfeeding, and immunosuppression are not contraindications or precautions to administration of Tdap.
Adverse Reactions Following Vaccination
DTaP
As with all injected vaccines, administration of DTaP may cause local reactions, such as pain, redness, or swelling. Local reactions have been reported in 20%–40% of children after the first three doses. Local reactions appear to be more frequent after the fourth and/or fifth doses. Mild systemic reactions such as drowsiness, fretfulness, and low-grade fever may also occur. Temperature of 101°F or higher is reported in 3%–5% of DTaP recipients. These reactions are self-limited and can be managed with symptomatic treatment with acetaminophen or ibuprofen. Moderate or severe systemic reactions (such as fever [105°F or higher], febrile seizures, persistent crying lasting 3 hours or longer, and hypotonic-hyporesponsive episodes) have been reported after administration of DTaP but occur less frequently than among children who received whole-cell DTP. Rates of these less common reactions vary by symptom and vaccine but generally occur in fewer than 1 in 10,000 doses. See the Pertussis chapter in the textbook Vaccines (Plotkin, Orenstein, and Offit eds., 2013) for a comprehensive review of DTaP adverse event data.
Information on adverse reactions following a full series of DTaP is also limited. Available data suggest a substantial increase in the frequency and magnitude of local reactions after the fourth and fifth doses. For example, swelling at the site of injection occurred in 2% of patients after the first dose of Tripedia, and in 29% following the fourth dose. Increases in the frequency of fever after the fourth dose have also been reported, although the increased frequencies of other systemic reactions (e.g., fretfulness, drowsiness, or decreased appetite) have not been observed. Further details on this issue can be found in a supplemental ACIP statement published in 2000 (MMWR 2000;49(No RR-13):1–8).
Swelling involving the entire thigh or upper arm has been reported after booster doses of certain acellular pertussis vaccines. The limb swelling may be accompanied by erythema, pain and fever. Although the swelling may interfere with walking, most children have no limitation of activity. The pathogenesis and frequency of substantial local reactions and limb swelling are not known, but these conditions appear to be self-limited and resolve without sequelae.
ACIP recommends that a fifth dose of DTaP be administered before a child enters school. It is not known whether children who experience entire limb swelling after a fourth dose of DTaP are at increased risk for this reaction after the fifth dose. Because of the importance of this dose in protecting a child during school years, ACIP recommends that a history of extensive swelling after the fourth dose should not be considered a contraindication to receipt of a fifth dose at school entry. Parents should be informed of the increase in reactogenicity that has been reported following the fourth and fifth doses of DTaP.
Tdap Adverse Reactions
- Local reactions (pain, redness, swelling)
- 21%-66%
- Temp of 101.4°F or higher
- 1.4%
- Adverse reactions occur at approximately the same rate as Td alone (without acellular pertussis vaccine)
Tdap
The safety of Tdap vaccines was evaluated as part of prelicensure studies. The most common adverse reaction following both brands of Tdap vaccine is a local reaction, such as pain (66%), redness (25%) or swelling (21%) at the site of injection. Temperature of 100.4°F or higher was reported by 1.4% of Tdap recipients and 1.1% of Td recipients. Tdap recipients also reported a variety of nonspecific systemic events, such as headache, fatigue and gastrointestinal symptoms. Local reactions, fever, and nonspecific systemic symptoms occurred at approximately the same rate in recipients of Tdap and the comparison group that received Td without acellular pertussis vaccine. No serious adverse events have been attributed to Tdap.
Vaccine Storage and Handling
DTaP, Td and Tdap vaccines should be stored at 35°–46°F (2°–8°C) at all times. The vaccines must never be frozen. Vaccine exposed to freezing temperature must not be administered and should be discarded. DTaP, Td and Tdap should not be used after the expiration date printed on the box or label.
Acknowledgment
The editors thank Drs. Jennifer Liang, Cindy Weinbaum, and Pedro Moro; and Stacey Martin, CDC for their assistance in updating this chapter.
Selected References
- American Academy of Pediatrics. Pertussis. In: Pickering L, Baker CJ, Kimberlin D, Long SS, eds Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:504-19.
- CDC. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-7):1-25.
- CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis. 2005 CDC Guidelines. MMWR 2005;54(No. RR-14):1-16.
- CDC. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-3):1-43.
- CDC. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-17):1-33.
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) Vaccine from the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR 2011;60(No.1):13-15.
- CDC. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine (Tdap) in Pregnant Women — Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2012; 62(07);131-135.
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant <12 months of age – Advisory Committee on Immunization Practices (ACIP), 2011.
- Cherry JD, The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 2005;115:1422-7.
- Edwards KM, Decker MD. Pertussis Vaccines. In: Plotkin SA, Orenstein, WA, and Offit PA, eds.Vaccines. 6th ed. China: Saunders; 2013.
- Greenberg DP. Pertussis in adolescents: increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr Infect Dis J 2005;24:721-8.
- Ward JI, Cherry JD, Chang SJ, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med 2005;353:1555-63.
- Woo EJ, Burwen DR, Gatumu SNM, et al. Extensive limb swelling after immunization: Reports to the Vaccine Adverse Event Reporting System. Clin Infect Dis 2003;37:351-8.
- Page last reviewed: November 15, 2016
- Page last updated: August 10, 2017
- Content source:


 ShareCompartir
ShareCompartir