Mumps
On this Page
Mumps is an acute viral illness. Parotitis and orchitis were described by Hippocrates in the 5th century BCE. In 1934, Johnson and Goodpasture showed that mumps could be transmitted from infected patients to rhesus monkeys and demonstrated that mumps was caused by a filterable agent present in saliva. This agent was later shown to be a virus. Mumps was a frequent cause of outbreaks among military personnel in the prevaccine era, and was one of the most common causes of aseptic meningitis and sensorineural deafness in childhood. During World War I, only influenza and gonorrhea were more common causes of hospitalization among soldiers. In 2006, a multistate mumps outbreak in the Midwest resulted in more than 6,000 reported cases. During 2009-2010, two large outbreaks occurred: one among Orthodox Jewish communities in the Northeast with 3,502 reported cases and the other on the U.S. Territory of Guam with 505 mumps cases reported.
Mumps
- Acute viral illness
- Parotitis and orchitis described by Hippocrates in 5th century BCE
- Viral etiology described by Johnson and Goodpasture in 1934
- Frequent cause of outbreaks among military personnel in prevaccine era
Mumps Virus
- Paramyxovirus
- RNA virus
- Rapidly inactivated by chemical agents, heat, and ultraviolet light
Mumps Pathogenesis
- Respiratory transmission of virus
- Replication in nasopharynx and regional lymph nodes
- Viremia 12 to 25 days after exposure with spread to tissues
- Multiple tissues infected during viremia
Mumps Clinical Features
- Incubation period 12 to 25 days
- Nonspecific prodrome of myalgia, malaise, headache, low-grade fever
- Parotitis in 9%-94%
- 15%-27% of infections asymptomatic in prevaccine era
Mumps Virus
Mumps virus is a paramyxovirus in the same group as parainfluenza and Newcastle disease virus. Parainfluenza and Newcastle disease viruses produce antibodies that cross-react with mumps virus. The virus has a single-stranded RNA genome.
The virus can be isolated or propagated in cultures of various human and monkey tissues and in embryonated eggs. It has been recovered from the saliva, cerebrospinal fluid, urine, blood, breastmilk, and infected tissues of patients with mumps.
Mumps virus is rapidly inactivated by formalin, ether, chloroform, heat, and ultraviolet light.
Pathogenesis
The virus is acquired by respiratory droplets. It replicates in the nasopharynx and regional lymph nodes. After 12 to 25 days a viremia occurs, which lasts from 3 to 5 days. During the viremia, the virus spreads to multiple tissues, including the meninges, and glands such as the salivary, pancreas, testes, and ovaries. Inflammation in infected tissues leads to characteristic symptoms of parotitis and aseptic meningitis.
Clinical Features
The incubation period of mumps is 12 to 25 days, but parotitis typically develops 16 to 18 days after exposure to mumps virus. The prodromal symptoms are nonspecific, and include myalgia, anorexia, malaise, headache, and low-grade fever.
Parotitis is the most common manifestation. Rates of classical parotitis among all age groups typically range from 31% to 65%, but in specific age groups can be as low as 9% or as high as 94% depending on the age and immunity of the group. Parotitis may be unilateral or bilateral, and any combination of single or multiple salivary glands may be affected. Parotitis tends to occur within the first 2 days and may first be noted as earache and tenderness on palpation of the angle of the jaw. Symptoms tend to decrease after one week and usually resolve after 10 days.
Before the introduction of the mumps vaccine in the United States in 1967, 15% to 27% of infections were asymptomatic. In the postvaccine era, it is difficult to estimate the number of asymptomatic infections, because it is unclear how vaccine modifies clinical presentation. Serious complications can occur in the absence of parotitis. Several articles discuss mumps symptoms as nonspecific or primarily respiratory, however, findings in these articles were based on serologies taken every six months or a year, so it is difficult to prove that the respiratory symptoms were because of mumps or that the symptoms occurred at the same time as the mumps infection.
Complications
Mumps Complications
- Orchitis
- 12%-66% in postpubertal males (prevaccine)
- 3%-10% (postvaccine)
- Pancreatitis
- 3.5% (prevaccine)
- Unilateral Deafness
- 1/20,000 (prevaccine)
- Death
- 2/10,000 from 1966-1971
- No deaths in recent U.S. outbreaks
Orchitis (testicular inflammation) is the most common complication in postpubertal males. In the prevaccine era, orchitis was reported in 12% to 66% of postpubertal males infected with mumps. In 60% to 83% of males with mumps orchitis, only one testis was affected. With mumps-associated orchitis, there is usually abrupt onset of testicular swelling, tenderness, nausea, vomiting, and fever. Pain and swelling may subside in 1 week, but tenderness may last for weeks. Sterility from mumps orchitis, even bilateral orchitis, occurred infrequently. In U.S. outbreaks in 2006 and 2009–2010 (the postvaccine era), rates of orchitis among postpubertal males have ranged from 3.3% to 10%. Orchitis usually occurs after parotitis, but it may precede it, begin simultaneously, or occur alone.
In the 2006 and 2009–2010 U.S. mumps outbreaks, oophoritis (ovarian inflammation) rates were 1% or lower among postpubertal females. It may mimic appendicitis. There is no relationship to impaired fertility.
In the prevaccine era, mumps accounted for approximately 10% of cases of symptomatic aseptic meningitis (inflammatory cells in cerebrospinal fluid resulting in headache or stiff neck). Men were afflicted three times as often as women. Aseptic meningitis resolves without sequelae in 3 to 10 days. Mumps encephalitis accounted for 36% of all reported encephalitis cases in the United States in 1967.
The incidence of mumps encephalitis is reported to range from 1 in 6,000 mumps cases (0.02%) to 1 in 300 mumps cases (0.3%).
Prior to the vaccine, pancreatitis was reported in 3.5% of persons infected with mumps in one community during a two year period and was described in case reports. Pancreatitis is infrequent, but occasionally occurs without parotitis; the hyperglycemia is transient and is reversible. Although single instances of diabetes mellitus have been reported, a causal relationship with mumps virus infection has yet to be conclusively demonstrated; many cases of temporal association have been described both in siblings and individuals, and outbreaks of diabetes have been reported a few months or years after outbreaks of mumps.
In the prevaccine era, mumps caused transient deafness in 4.1% of infected adult males in a military population. Permanent unilateral deafness caused by mumps occurred in 1 of 20,000 infected persons; bilateral, severe hearing loss was very rare.
In the postvaccine era, among all persons infected with mumps, reported rates of meningitis, encephalitis, pancreatitis, and deafness have all been less than 1%. Permanent sequelae such as paralysis, seizures, cranial nerve palsies, and hydrocephalus occurred very rarely, even in the prevaccine era. Although, in the United States during 1966–1971 there were two deaths per 10,000 reported mumps cases, there were no mumps-related deaths in recent U.S. outbreaks.
Laboratory Diagnosis
Mumps Laboratory Diagnosis
- rRT-PCR
- Culture
- Serology
The diagnosis of mumps is usually suspected based on clinical manifestations, in particular the presence of parotitis. However, if mumps is suspected, laboratory testing should be performed. Acute mumps infection can be detected by the presence of serum mumps IgM, a significant rise in IgG antibody titer in acute and convalescent-phase serum specimens, IgG seroconversion, positive mumps virus culture, or detection of virus by real-time reverse transcriptase polymerase chain reaction (rRT-PCR). However, in both unvaccinated and vaccinated persons, false positive results can occur because assays may be affected by other diagnostic entities that cause parotitis. In addition, laboratory confirming the diagnosis of mumps in highly vaccinated populations may be challenging, and serologic tests should be interpreted with caution because false negative results in vaccinated persons (i.e., a negative serologic test in a person with true mumps) are common. With previous contact with mumps virus either through vaccination (particularly with two doses) or natural infection, serum mumps IgM test results may be negative; IgG test results may be positive at the initial blood draw; and viral detection in rRT-PCR or culture may have low yield if the buccal swab is collected more than three days after parotitis onset. Therefore, mumps cases should not be ruled out by negative laboratory results.
Mumps virus can be isolated from the parotid duct, other affected salivary gland ducts, the throat, from urine, and from cerebrospinal fluid (CSF). The preferred sample for viral isolation is a swab from the parotid duct, or the duct of another affected salivary gland. Collection of viral samples from persons suspected of having mumps is strongly recommended. Clinical specimens should ideally be obtained within three days and not more than eight days after parotitis onset. Mumps virus can also be detected by real-time reverse transcriptase polymerase chain reaction (rRT-PCR). Molecular typing is recommended because it provides important epidemiologic information, including transmission pathways of mumps strains circulating in the United States and it is a tool for distinguishing wild-type mumps virus from vaccine virus.
Serology is the simplest method for confirming mumps virus infection and enzyme immunoassay (EIA), is the most commonly used test. EIA is widely available and is more sensitive than other serologic tests. It is available for both IgM and IgG. In unvaccinated persons, IgM antibodies usually become detectable during the first 5 days of illness, reach a peak about a week after onset, and remain elevated for several weeks or months. However, as with measles and rubella, mumps IgM may be transient or missing in persons who have had any doses of mumps-containing vaccine. Sera should be collected as soon as possible after symptom onset for IgM testing or as the acute-phase specimen for IgG seroconversion. Convalescent-phase sera should be collected 2 weeks later. A negative serologic test, especially in a vaccinated person, should not be used to rule out a mumps diagnosis because the tests are not sensitive enough to detect infection in all persons with clinical illness. In the absence of another diagnosis, a person meeting the clinical case definition should be reported as a suspect mumps case. Additional information about specimen collection and shipping for mumps specimens may be obtained from the CDC mumps website.
Epidemiology
Mumps Epidemiology
- Reservoir
- human
- asymptomatic infections may transmit
- Transmission
- airborne
- direct contact with droplet nuclei or saliva
- Temporal pattern
- peak in late winter and spring
- Communicability
- several days before and after onset of parotitis
Occurrence
Mumps occurs worldwide.
Reservoir
Mumps is a human disease. Although persons with asymptomatic or nonclassical infection can transmit the virus, no carrier state is known to exist.
Transmission
Mumps is spread through airborne transmission or by direct contact with infected droplet nuclei or saliva.
Temporal Pattern
Mumps incidence peaks predominantly in late winter and spring, but the disease has been reported throughout the year.
Communicability
Contagiousness is similar to that of influenza and rubella, but is less than that for measles or varicella. Although mumps virus has been isolated from seven days before, through 11–14 days after parotitis onset, the highest percentage of positive isolations and the highest virus loads occur closest to parotitis onset and decrease rapidly thereafter. Mumps is therefore most infectious in the several days before and after parotitis onset. Most transmission likely occurs several days before and after parotitis onset. Transmission also likely occurs from persons with asymptomatic infections and from persons with prodromal symptoms.
Secular Trends in the United States
Mumps - United States, 1968-2011
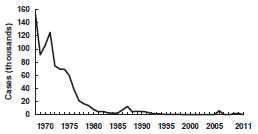
Source: National Notifiable Disease Surveillance System, CDC
Mumps became a nationally reportable disease in the United States in 1968. However, an estimated 212,000 cases occurred in the United States in 1964. Following vaccine licensure, reported mumps decreased rapidly. Approximately 3,000 cases were reported annually in 1983–1985 (1.3–1.55 cases per 100,000 population).
In 1986 and 1987, there was a relative resurgence of mumps, which peaked in 1987, when 12,848 cases were reported. The highest incidence of mumps during the resurgence was among older school-age and college-age youth (10–19 years of age), who were born before routine mumps vaccination was recommended. Mumps incidence in this period correlated with the absence of comprehensive state requirements for mumps immunization. Several mumps outbreaks among highly vaccinated school populations were reported, indicating that high coverage with a single dose of mumps vaccine did not always prevent disease transmission, probably because of vaccine failure.
Since 1989 when two doses of MMR vaccine were recommended for school-aged children for improved measles control, the number of reported mumps cases steadily declined, from 5,712 cases in 1989 to 258 cases in 2004. In 2006, the United States experienced a multi-state outbreak involving 6,584 reported cases of mumps. This resurgence predominantly affected Midwestern college students with the highest attack rates occurring among those living in dormitories. In the following two years, the number of reported cases returned to usual levels, and outbreaks involved fewer than 20 cases.
Mumps - United States, 1980-2011
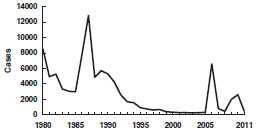
Source: National Notifiable Disease Surveillance System, CDC
Beginning in June 2009, the largest U.S. mumps outbreak since 2006 has occurred. The index case was an 11 year old male infected in the United Kingdom, where approximately 7,400 reports of laboratory-confirmed mumps were received by the Health Protection Agency in 2009. A total of 3,502 outbreak-related cases were reported, primarily from New York. The outbreak was confined primarily to Orthodox Jewish communities, with less than 3% of cases occurring among persons outside these communities. The largest percentage of cases (53%) occurred among persons aged 5–17 years, and 71% of the patients were male. Among the patients for whom vaccination status was reported, 90% had received at least 1 dose of mumps-containing vaccine, and 76% had received 2 doses.
From December 2009, through December 2010, the U.S. Territory of Guam also experienced an outbreak, with 505 mumps cases reported; the median age was 12 years. Of the 287 school-aged children aged 6–18 years with reported mumps, 270 (94%) had received at least two doses of MMR vaccine, 8 (3%) had received one dose, 2 (1%) were unvaccinated, and 7 (2%) had unknown vaccination status. Two-dose MMR vaccine coverage in the most highly affected schools ranged from 99.3%–100%.
Like the mumps outbreaks that occurred in 2006, much of the 2009-2010 outbreaks occurred in congregate settings, where prolonged, close contact among persons facilitated transmission. Although school settings and large household sizes likely promoted transmission, the high vaccination coverage in the affected communities likely limited the size of the outbreaks. In addition, high vaccination coverage and less intense exposures in surrounding communities are the most plausible reasons that the few cases outside of the affected communities did not cause other outbreaks.
In 2011, there were 404 cases of mumps reported, and in 2012 there were 229 cases reported.
See more information about the clinical case definition, clinical classification and epidemiologic classification of mumps.
Mumps Vaccine
Characteristics
Mumps Vaccine
- Composition
- Live virus (Jeryl Lynn strain)
- Effectiveness
- 88% (Range, 66%-95%) – 2 doses
- Duration of Immunity
- lifelong
- Schedule
- at least 1 dose
- should be administered with measles and rubella (MMR) or with measles, rubella and varicella (MMRV)
- Single-antigen vaccine not available in the United States
Mumps virus was isolated in 1945, and an inactivated vaccine was developed in 1948. This vaccine produced only short-lasting immunity, and its use was discontinued in the mid-1970s. The currently used Jeryl Lynn strain of live attenuated mumps virus vaccine was licensed in December 1967. The vaccine was recommended for routine use in the United States in 1977.
Mumps vaccine is available combined with measles and rubella vaccines (as MMR), or combined with measles, rubella, and varicella vaccine as MMRV (ProQuad). Single-antigen mumps vaccine is not available in the United States.
Mumps vaccine is prepared in chick embryo fibroblast tissue culture. MMR and MMRV are supplied as a lyophilized (freeze-dried) powder and are reconstituted with sterile, preservative-free water. The vaccine contains small amounts of human albumin, neomycin, sorbitol, and gelatin.
Immunogenicity and Vaccine Efficacy
Mumps vaccine produces an inapparent, or mild, noncommunicable infection. Approximately 94% (89% to 97%) of recipients of a single dose develop measurable mumps antibody. Seroconversion rates are similar for single antigen mumps vaccine, MMR, and MMRV. Postlicensure studies determined that one dose of mumps or MMR vaccine was 78% (49% to 92%) effective. Two dose mumps vaccine effectiveness is 88% (66% to 95%).
Vaccination Schedule and Use
Mumps (MMR) Vaccine Indications
- One dose (as MMR) for preschool-age children 12 months of age and older and persons born during or after 1957 not at high risk of mumps exposure
- Second dose (as MMR) for school-age children and adults at high risk of mumps exposure (i.e., healthcare personnel, international travelers and students at post-high school educational institutions)
In 1977, one dose of mumps-containing vaccine was routinely recommended for all children 12 months of age and older. In 1989, children began receiving two doses of mumps vaccine because of the implementation of a two-dose measles vaccination policy using the combined measles, mumps, and rubella (MMR) vaccine. In 2006, a two-dose mumps vaccine policy was recommended for school-aged children, students at post high school educational institutions, healthcare personnel, and international travelers.
The first dose of mumps-containing vaccine should be given on or after the first birthday. Mumps-containing vaccine given before 12 months of age should not be counted as part of the series. Children vaccinated with mumps-containing vaccine before 12 months of age should be revaccinated with two doses of MMR vaccine, the first of which should be administered when the child is at least 12 months of age.
The second dose should be given routinely at age 4 through 6 years, before a child enters kindergarten or first grade. The recommended health visit at age 11 or 12 years can serve as a catch-up opportunity to verify vaccination status and administer MMR vaccine to those children who have not yet received two doses of MMR. The second dose of MMR may be administered as soon as 4 weeks (i.e., 28 days) after the first dose. The combined MMR vaccine is recommended for both doses to ensure immunity to all three viruses.
Only doses of vaccine with written documentation of the date of receipt should be accepted as valid. Self-reported doses or a parental report of vaccination is not considered adequate documentation. A clinician should not provide an immunization record for a patient unless that clinician has administered the vaccine or has seen a record that documents vaccination. Persons who lack adequate documentation of vaccination or other acceptable evidence of immunity should be vaccinated. Vaccination status and receipt of all vaccinations should be documented in the patient’s permanent medical record and in a vaccination record held by the individual.
MMRV is approved by the Food and Drug Administration for children 12 months through 12 years of age (that is, until the 13th birthday). MMRV should not be administered to persons 13 years of age or older.
For the first dose of measles, mumps, rubella, and varicella vaccines at age 12 through 47 months, either separate MMR and varicella vaccines or MMRV vaccine may be used. Providers who are considering administering MMRV vaccine should discuss the benefits and risks of both vaccination options with the parents or caregivers. Unless the parent or caregiver expresses a preference for MMRV vaccine, ACIP recommends that MMR vaccine and varicella vaccine should be administered for the first dose in this age group (see Measles chapter for more information). For the second dose of measles, mumps, rubella, and varicella vaccines at any age (15 months through 12 years) and for the first dose at 48 months of age or older, use of MMRV vaccine generally is preferred over separate injections of its equivalent component vaccines (i.e., MMR vaccine and varicella vaccine).
Mumps Immunity
Mumps Immunity
- Born before 1957
- Serologic evidence of mumps immunity
- Laboratory confirmation of disease
- Documentation of adequate vaccination
Generally, persons can be considered immune to mumps if they were born before 1957, have serologic evidence of mumps immunity or laboratory confirmation of disease, or have written documentation of adequate vaccination for mumps at age 12 months or older. Demonstration of mumps IgG antibody by any commonly used serologic assay is acceptable evidence of mumps immunity. Persons who have an “equivocal” serologic test result should be considered susceptible to mumps.
For unvaccinated health-care personnel born before 1957 who lack laboratory evidence of measles, mumps and/or rubella immunity or laboratory confirmation of disease, healthcare facilities should consider vaccination with two doses of MMR vaccine at the appropriate interval (for measles and mumps) and one dose of MMR vaccine (for rubella), respectively. For unvaccinated personnel born before 1957 who lack laboratory evidence of measles, mumps and/or rubella immunity or laboratory confirmation of disease, healthcare facilities should recommend two doses of MMR vaccine during an outbreak of measles or mumps and one dose during an outbreak of rubella.
Postexposure Prophylaxis
Immune globulin (IG) is not effective postexposure prophylaxis. Vaccination after exposure is not harmful and may possibly avert later disease.
Contraindications and Precautions to Vaccination
MMR Vaccine Contraindications and Precautions
- History of anaphylactic reactions to neomycin
- History of severe allergic reaction to any component of the vaccine
- Pregnancy
- Immunosuppression
- Moderate or severe acute illness
- Recent blood product
- Personal or family (i.e., sibling or parent) history of seizures of any etiology (MMRV only)
Measles and Mumps Vaccines and Egg Allergy
- Measles and mumps viruses grown in chick embryo fibroblast culture
- Studies have demonstrated safety of MMR in egg allergic children
- Vaccinate without testing
Contraindications for MMR and MMRV vaccines include history of anaphylactic reactions to neomycin, history of severe allergic reaction to any component of the vaccine, pregnancy, and immunosuppression.
In the past, persons with a history of anaphylactic reactions following egg ingestion were considered to be at increased risk of serious reactions after receipt of measles- or mumps-containing vaccines, which are produced in chick embryo fibroblasts. However, data suggest that most anaphylactic reactions to measles- and mumps-containing vaccines are not associated with hypersensitivity to egg antigens but to other components of the vaccines (such as gelatin). The risk for serious allergic reactions such as anaphylaxis following receipt of these vaccines by egg-allergic persons is extremely low, and skin-testing with vaccine is not predictive of allergic reaction to vaccination. As a result, MMR may be administered to egg-allergic children without prior routine skin-testing or the use of special protocols.
MMR vaccine does not contain penicillin. A history of penicillin allergy is not a contraindication to MMR vaccination.
Pregnant women should not receive mumps vaccine, although the risk is theoretical. There is no evidence that mumps vaccine virus causes fetal damage. Pregnancy should be avoided for 4 weeks after vaccination with MMR vaccine.
Persons with immunodeficiency or immunosuppression resulting from leukemia, lymphoma, generalized malignancy, immune deficiency disease, or immunosuppressive therapy should not be vaccinated. However, treatment with low-dose (less than 2 mg/kg/day), alternate-day, topical, or aerosolized steroid preparations is not a contraindication to mumps vaccination. Persons whose immunosuppressive therapy with steroids has been discontinued for 1 month (3 months for chemotherapy) may be vaccinated. See Measles chapter for additional details on vaccination of immunosuppressed persons, including those with HIV infection.
Persons with moderate or severe acute illness should not be vaccinated until the illness has improved. Minor illness (e.g., otitis media, mild upper respiratory infections), concurrent antibiotic therapy, and exposure or recovery from other illnesses are not contraindications to mumps vaccination.
Receipt of antibody-containing blood products (e.g., immune globulin, whole blood or packed red blood cells, intravenous immune globulin) may interfere with seroconversion following mumps vaccination. Vaccine should be given 2 weeks before, or deferred for at least 3 months following, administration of an antibody-containing blood product. See Chapter 2, General Recommendations on Immunization, for details.
MMR Adverse Events
- Fever
- not common
- Rash
- not common
- Joint symptoms
- 25%
- Orchitis
- not common
- Parotitis
- rare
- CNS reactions
- 1/800,000 doses
A personal or family (i.e., sibling or parent) history of seizures of any etiology is a precaution for MMRV vaccination. Studies suggest that children who have a personal or family history of febrile seizures or family history of epilepsy are at increased risk for febrile seizures compared with children without such histories. Children with a personal or family history of seizures of any etiology generally should be vaccinated with MMR vaccine and varicella vaccine because the risks for using MMRV vaccine in this group of children generally outweigh the benefits. A family history of diabetes is not a contraindication to vaccination with MMR vaccine.
Adverse Events Following Vaccination
Most adverse events reported following MMR vaccine (such as fever, rash, and joint symptoms) are attributable to the measles or rubella components. No adverse reactions were reported in large-scale field trials. Subsequently, parotitis and fever have been reported rarely. A few cases of orchitis (all suspect) also have been reported.
Rare cases of central nervous system (CNS) dysfunction, including cases of deafness, within 2 months of mumps vaccination have been reported. The Institute of Medicine (1994) concluded that evidence is inadequate to accept or reject a causal relationship between the Jeryl Lynn strain of mumps vaccine and aseptic meningitis, encephalitis, sensorineural deafness, or orchitis.
Adverse Reactions Following Vaccination
MMR Adverse Reactions
- Allergic reactions (rash, pruritis, purpura)
- not common
- CNS reactions
- 1/800,000 doses
Allergic reactions, including rash, pruritus, and purpura, have been temporally associated with vaccination, but these are transient and generally mild. The calculated incidence of CNS reactions is approximately one per 800,000 doses of Jeryl Lynn strain of mumps vaccine virus.
See the Measles and Varicella chapters for information about adverse reactions following MMRV vaccine.
Vaccine Storage and Handling
MMR vaccine can be stored either in the freezer or the refrigerator and should be protected from light at all times. MMRV vaccine should be stored frozen between -58°F and +5°F (-50°C and -15°C). When MMR vaccine is stored in the freezer, the temperature should be the same as that required for MMRV, between -58°F and +5°F (-50°C and -15°C). Storing MMR in the freezer with MMRV may help prevent inadvertent storage of MMRV in the refrigerator.
Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
Acknowledgments
The editors thank Amy Parker Fiebelkorn, CDC for her assistance in updating this chapter.
Selected References
- Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, et al. Mumps Outbreak in Orthodox Jewish Communities in the United States. New Engl J Med. 2012 Nov 1;367(18):1704-13.
- CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR 2013:62(No. 4): 1-34.
- CDC. General recommendations on immunization. recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2011; 60(RR-2);1-61.
- CDC. Notice to readers: Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the control and elimination of mumps. MMWR 2006;55:629–30.
- CDC. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8):1–57.
- CDC. Mumps surveillance: January 1972–June 1974. Atlanta, GA: U.S. Department of Health, Education, and Welfare, Public Health Service, 1974.
- CDC. Mumps vaccine. 1977. MMWR 26(48):393–94.
- CDC. Update: Mumps outbreak—New York and New Jersey, June 2009–January 2010. MMWR 2010;59:125–9.
- CDC. Updated recommendations for isolation of persons with mumps. MMWR 2008. 57(40):1103–5.
- CDC. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-3):1–12.
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008 Apr 10;358(15):1580-9.
- Dayan GH and Rubin S. Mumps outbreaks in unvaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Clin Infect Dis 2008;47:1458-67.
- Everberg G. Deafness following mumps. Acta Otolaryngol 1957;48(5–6):397–403.
- Falk WA, et al. The epidemiology of mumps in southern Alberta 1980–1982. Am J Epidemiol 1989;130(4):736–49.
- Hirsh BS, Fine PEM, Kent WK, et al. Mumps outbreak in a highly vaccinated population. J Pediatr 1991;119:187–93.
- Kutty PK, et al. Guidance for isolation precautions for mumps in the United States: a review of the scientific basis for policy change. Clin Infect Dis 2010;50(12):1619–28.
- Lee CM. Primary virus mastitis from mumps. Va Med Mon (1918) 1946;73:327.
- Litman N and Baum SG. Mumps virus. In: Mandell GL, Bennett JE, and Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsiever; 2010. p. 2201–06.
- Morrison JC, et al. Mumps oophoritis: a cause of premature menopause. Fertil Steril 1975;26(7)655–9.
- Miller HG, Stanton JB, Gibbons JL. Parainfectious encephalomyelitis and related syndromes; a critical review of neurological complications of certain specific fevers. Q J Med 1956;25:427–505.
- Nelson GE, Aguon A, Valencia E, Oliva R, Guerrero ML, Reyes R, et al. Epidemiology of a Mumps Outbreak in a Highly Vaccinated Island Population and Use of a Third Dose of Measles-Mumps-Rubella Vaccine for Outbreak Control- Guam 2009-2010. Pediatr Infect Dis J. 2012 Oct 24.
- Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012 Dec;130(6):e1567-74.
- Orenstein WA, Hadler S, Wharton M. Trends in vaccine-preventable diseases. Semin Pediatr Infect Dis 1997;8:23–33.
- Philip RN, Reinhard KR, Lackman DB. Observations on a mumps epidemic in a virgin population. Am J Hyg 1959;69(2):91–111.
- Plotkin SA, Rubin SA. Mumps vaccine. In: Plotkin SA, Orenstein, WA, Offit PA, eds. Vaccines. 5th ed. China: Saunders;2008:435–65.
- Rambar AC. Mumps; Use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child 1946;71:1–13.
- Reed D, et al. A mumps epidemic on St. George Island, Alaska. JAMA 1967; 199(13):113–7.
- Russell RR and Donald JC. The neurological complications of mumps. Br Med J 1958;2(5087):27–30.
- Taparelli F, et al. Isolation of mumps virus from vaginal secretions in association with oophoritis. J Infect 1988;17(3)255–8.
- Van Loon FPL, Holmes SJ, Sirotkin BI, et al. Mumps surveillance — United States, 1988–1993. In: CDC Surveillance Summaries, August 11, 1995. MMWR 1995;44(No. SS-3):1-14.
- Veghelyi PV. Secondary pancreatitis. Am J Dis Child 1947;74(1):45–51.
- Weaver RJ and Petry TN. Mumps mastitis in the nursing female, with a case report. J Indiana State Med Assoc 1958;51(5)644–5.
- Werner CA. Mumps orchitis and testicular atrophy; occurrence. Ann Intern Med 1950;32(6):1066–74.
- Witte CL and Schanzer B. Pancreatitis due to mumps. JAMA 1968;203(12):1068–9.
- Page last reviewed: November 15, 2016
- Page last updated: July 30, 2015
- Content source:


 ShareCompartir
ShareCompartir