Hepatitis B
On this Page
Hepatitis B
- Epidemic jaundice described by Hippocrates in 5th century BCEs
- Jaundice reported among recipients of human serum and yellow fever vaccines in 1930s and 1940s
- Australia antigen described in 1965
- Serologic tests developed in 1970s
Viral hepatitis is a term commonly used for several clinically similar yet etiologically and epidemiologically distinct diseases. Hepatitis A (formerly called infectious hepatitis) and hepatitis B (formerly called serum hepatitis) have been recognized as separate entities since the early 1940s and can be diagnosed with specific serologic tests. Delta hepatitis is an infection dependent on the hepatitis B virus (HBV). It may occur as a coinfection with acute HBV infection or as superinfection of an HBV carrier.
Epidemic jaundice was described by Hippocrates in the 5th century BCE. The first recorded cases of “serum hepatitis,” or hepatitis B, are thought to be those that followed the administration of smallpox vaccine containing human lymph to shipyard workers in Germany in l883. In the early and middle parts of the 20th century, serum hepatitis was repeatedly observed following the use of contaminated needles and syringes. The role of blood as a vehicle for virus transmission was further emphasized in 1943, when Beeson described jaundice that had occurred in seven recipients of blood transfusions. Australia antigen, later called hepatitis B surface antigen (HBsAg), was first described in 1965, and the Dane particle (complete hepatitis B virion) was identified in 1970. Identification of serologic markers for HBV infection followed, which helped clarify the natural history of the disease. Ultimately, HBsAg was prepared in quantity and now comprises the immunogen in highly effective vaccines for prevention of HBV infection.
Hepatitis B Virus
Hepatitis B Virus
- Hepadnaviridae family (DNA)
- Numerous antigenic components
- Humans are only known host
- May retain infectivity for more than 7 days at room temperature
Hepatitis B Virus Infection
- More than 350 million chronically infected worldwide
- Established cause of chronic hepatitis and cirrhosis
- Human carcinogen—cause of up to 50% of hepatocellular carcinomas
- More than 600,000 deaths worldwide in 2002
Hepatitis B Virus
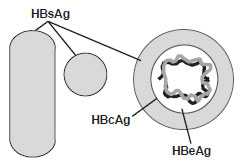
HBV is a small, double-shelled virus in the family Hepadnaviridae. Other Hepadnaviridae include duck hepatitis virus, ground squirrel hepatitis virus, and woodchuck hepatitis virus. The virus has a small circular DNA genome that is partially double-stranded. HBV contains numerous antigenic components, including HBsAg, hepatitis B core antigen (HBcAg), and hepatitis B e antigen (HBeAg). Humans are the only known host for HBV, although some nonhuman primates have been infected in laboratory conditions. HBV is relatively resilient and, in some instances, has been shown to remain infectious on environmental surfaces for more than 7 days at room temperature.
An estimated 2 billion persons worldwide have been infected with HBV, and more than 350 million persons have chronic, lifelong infections. HBV infection is an established cause of acute and chronic hepatitis and cirrhosis. It is the cause of up to 50% of hepatocellular carcinomas (HCC). The World Health Organization estimated that more than 600,000 persons died worldwide in 2002 of hepatitis B-associated acute and chronic liver disease.
Several well-defined antigen–antibody systems are associated with HBV infection. HBsAg, formerly called Australia antigen or hepatitis-associated antigen, is an antigenic determinant found on the surface of the virus. It also makes up subviral 22-nm spherical and tubular particles. HBsAg can be identified in serum 30 to 60 days after exposure to HBV and persists for variable periods. HBsAg is not infectious. Only the complete virus (Dane particle) is infectious. During replication, HBV produces HBsAg in excess of that needed for production of Dane particles. HBsAg is antigenically heterogeneous, with a common antigen (designated a) and 2 pairs of mutually exclusive antigens (d, y, w [including several subdeterminants] and r), resulting in 4 major subtypes: adw, ayw, adr and ayr. The distribution of subtypes varies geographically; because of the common “a” determinant, protection against one subtype appears to confer protection against the other subtypes, and no differences in clinical features have been related to subtype.
HBcAg is the nucleocapsid protein core of HBV. HBcAg is not detectable in serum by conventional techniques, but it can be detected in liver tissue of persons with acute or chronic HBV infection. HBeAg, a soluble protein, is also contained in the core of HBV. HBeAg is detected in the serum of persons with high virus titers and indicates high infectivity. Antibody to HBsAg (anti-HBs) develops during convalescence after acute HBV infection or following hepatitis B vaccination. The presence of anti-HBs indicates immunity to HBV. (Anti-HBs is sometimes referred to as HBsAb, but use of this term is discouraged because of potential confusion with HBsAg.) Antibody to HBcAg (anti-HBc) indicates infection with HBV at an undefined time in the past. IgM class antibody to HBcAg (IgM anti-HBc) indicates recent infection with HBV. Antibody to HBeAg (anti-HBe) becomes detectable when HBeAg is lost and is associated with low infectivity of serum.
Genotype classification based on sequencing of genetic material has been introduced and is becoming the standard: HBV is currently classified into 8 main genotypes (A–H). HBV genotypes are associated with the modes of HBV transmission (vertical vs. horizontal) and with the risk of certain outcomes of chronic infection, such as cirrhosis and HCC. In Alaska, HBV genotype F is associated with HCC in young children as well as adults younger than 30 years of age. In Asia as well as Alaska, HBV genotype C has been associated with a significantly higher risk of HCC than other genotypes.
Clinical Features
Hepatitis B Clinical Features
- Incubation period 45-160 days (average 120 days)
- Nonspecific prodrome of malaise, fever, headache, myalgia
- Illness not specific for hepatitis B
- At least 50% of infections asymptomatic
The clinical course of acute hepatitis B is indistinguishable from that of other types of acute viral hepatitis. The incubation period ranges from 45 to 160 days (average,120 days). Clinical signs and symptoms occur more often in adults than in infants or children, who usually have an asymptomatic acute course. However, approximately 50% of adults who have acute infections are asymptomatic.
The preicteric, or prodromal phase from initial symptoms to onset of jaundice usually lasts from 3 to l0 days. It is nonspecific and is characterized by insidious onset of malaise, anorexia, nausea, vomiting, right upper quadrant abdominal pain, fever, headache, myalgia, skin rashes, arthralgia and arthritis, and dark urine, beginning 1 to 2 days before the onset of jaundice. The icteric phase is variable but usually lasts from l to 3 weeks and is characterized by jaundice, light or gray stools, hepatic tenderness and hepatomegaly (splenomegaly is less common). During convalescence, malaise and fatigue may persist for weeks or months, while jaundice, anorexia, and other symptoms disappear.
Most acute HBV infections in adults result in complete recovery with elimination of HBsAg from the blood and the production of anti-HBs, creating immunity to future infection.
Hepatitis B Complications
- Fulminant hepatitis
- Hospitalization
- Cirrhosis
- Hepatocellular carcinoma
- Death
Chronic Hepatitis B Virus Infection
- Responsible for most mortality
- Overall risk 5% among adults
- Higher risk with early infection
- Often asymptomatic
Complications
While most acute HBV infections in adults result in complete recovery, fulminant hepatitis occurs in about 1% to 2% of acutely infected persons. About 200 to 300 Americans die of fulminant disease each year (case-fatality rate 63% to 93%). Although the consequences of acute HBV infection can be severe, most of the serious complications associated with HBV infection are due to chronic infection.
Chronic HBV Infection
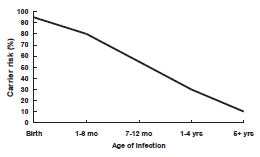
The proportion of patients with acute HBV Infection who progress to chronic infection varies with age and immune status. As many as 90% of infants who acquire HBV infection from their mothers at birth or in infancy become chronically infected. Of children who become infected with HBV between 1 year and 5 years of age, 30% to 50% become chronically infected. By adulthood, the risk of acquiring chronic HBV infection is approximately 5%. Acute HBV progresses to chronic HBV in approximately 40% of hemodialysis patients and up to 20% of patients with immune deficiencies.
Persons with chronic infection are often asymptomatic and may not be aware that they are infected; however, they are capable of infecting others and have been referred to as carriers. Chronic infection is responsible for most HBV-related morbidity and mortality, including chronic hepatitis, cirrhosis, liver failure, and hepatocellular carcinoma. Approximately 25% of persons with chronic HBV infection die prematurely from cirrhosis or liver cancer. Chronic active hepatitis develops in more than 25% of carriers and often results in cirrhosis. An estimated 3,000 to 4,000 persons die of hepatitis B-related cirrhosis each year in the United States. Persons with chronic HBV infection are at 12 to 300 times higher risk of hepatocellular carcinoma than noncarriers. An estimated 1,000 to 1,500 persons die each year in the United States of hepatitis B-related liver cancer.
Risk of Chronic HBV Carriage by Age of Infection
Laboratory Diagnosis
Diagnosis is based on clinical, laboratory, and epidemiologic findings. HBV infection cannot be differentiated on the basis of clinical symptoms alone, and definitive diagnosis depends on the results of serologic testing. Serologic markers of HBV infection vary depending on whether the infection is acute or chronic.
HBsAg is the most commonly used test for diagnosing acute HBV infections or detecting carriers. HBsAg can be detected as early as 1 or 2 weeks and as late as 11 or 12 weeks after exposure to HBV when sensitive assays are used. The presence of HBsAg indicates that a person is infectious, regardless of whether the infection is acute or chronic.
Anti-HBc (core antibody) develops in all HBV infections, appears shortly after HBsAg in acute disease, and indicates HBV infection at some undefined time in the past. Anti-HBc only occurs after HBV infection and does not develop in persons whose immunity to HBV is from vaccine. Anti-HBc generally persists for life and is not a serologic marker for acute infection.
IgM anti-HBc appears in persons with acute disease about the time of illness onset and indicates recent infection with HBV. IgM anti-HBc is generally detectable 4 to 6 months after onset of illness and is the best serologic marker of acute HBV infection. A negative test for IgM-anti-HBc together with a positive test for HBsAg in a single blood sample identifies a chronic HBV infection. HBV DNA assays are used to monitor response to treatment, assess the likelihood of maternal-to-child transmission of HBV, and to detect the presence of occult HBV infection (i.e. infection in someone who tests HBsAg negative).
HBeAg is a useful marker associated strongly with the number of infective HBV particles in the serum and a higher risk of infectivity.
| Tests | Results | Interpretation |
|---|---|---|
| HBsAg | Negative | Susceptible |
| anti-HBc | Negative | Susceptible |
| anti-HBs | Negative | Susceptible |
| HBsAg | Negative | Immune due to vaccination |
| anti-HBc | Negative | Immune due to vaccination |
| anti-HBs | Positive with ≥10mIU/mL* | Immune due to vaccination |
| HBsAg | Negative | Immune due to natural infection |
| anti-HBc | Positive | Immune due to natural infection |
| anti-HBs | Positive | Immune due to natural infection |
| HBsAg | Positive | Acutely infected |
| anti-HBc | Positive | Acutely infected |
| IgM anti-HBc | Positive | Acutely infected |
| anti-HBs | Negative | Acutely infected |
| HBsAg | Positive | Chronically infected |
| anti-HBc | Positive | Chronically infected |
| IgM anti-HBc | Negative | Chronically infected |
| anti-HBs | Negative | Chronically infected |
| HBsAg | Negative | Four interpretations possible† |
| anti-HBc | Positive | Four interpretations possible† |
| anti-HBs | Negative | Four interpretations possible† |
*Postvaccination testing, when it is recommended, should be performed 1-2 months following dose #3.
† 1. May be recovering from acute HBV infection.
2. May be distantly immune and test is not sensitive enough to detect a very low level of anti-HBs in serum.
3. May be susceptible with a false positive anti-HBc.
4. May be chronically infected and have an undetectable level of HBsAg present in the serum.
Anti-HBs (surface antibody) is a protective, neutralizing antibody. The presence of anti-HBs following acute HBV infection generally indicates recovery and immunity against reinfection. Anti-HBs can also be acquired as an immune response to hepatitis B vaccine or passively transferred by administration of hepatitis B immune globulin (HBIG). With enzyme immunoassay (EIA), the manufacturer’s recommended positive should be considered an appropriate measure of immunity. The level of anti-HBs may also be expressed in milli-international units/mL (mIU/mL). Ten mIU/mL is considered to indicate a protective level of immunity.
Medical Management
There is no specific therapy for acute HBV infection. Treatment is supportive.
Two major groups of antiviral treatment have been licensed for the treatment of chronic HBV infection in many countries. These include interferon alpha (IFNa, or PEG-IFNa) and nucleoside or nucleotide analogues such as lamivudine, adefovir, entecavir telbivudine, and tenofovir. Many other drugs are being evaluated. Although the decision to treat and choosing the appropriate therapy remain challenging, considerable progress has been made in the treatment of persons with chronic HBV infection. Patients generally are considered for treatment when they have HBV DNA levels above 2000 IU/ml, serum alanine aminotransferase levels above the upper limit of normal, and severity of liver disease assessed by liver biopsy (or non-invasive markers once validated in HBV-infected patients) showing moderate to severe active necroinflammation and/or at least moderate fibrosis using a standardized scoring system. The majority of patients will require prolonged treatment in order to maintain suppression of viral replication. Consequently, treatment costs in both developing and developed countries are currently prohibitively high. The efficacy of combination therapy will have to be studied further, but it is likely to diminish the occurrence of virus mutants resistant to treatment. Medications have significant side effects that require careful monitoring.
Persons with acute or chronic HBV infections should prevent their blood and other potentially infective body fluids from contacting other persons. They should not donate blood or share toothbrushes or razors with household members.
In the hospital setting, patients with HBV infection should be managed with standard precautions.
Epidemiology
Hepatitis B Epidemiology
- Reservoir
- human
- Transmission
- bloodborne
- subclinical cases transmit
- Communicability
- 1-2 months before and after onset of symptoms
- persons with either acute or chronic HBV infection with HBsAg present in blood
Reservoir
Although other primates have been infected in laboratory conditions, HBV infection affects only humans. No animal or insect hosts or vectors are known to exist.
Transmission
The virus is transmitted by parenteral or mucosal exposure to HBsAg-positive body fluids from persons who have acute or chronic HBV infection. The highest concentrations of virus are in blood and serous fluids; lower titers are found in other fluids, such as saliva, tears, urine, and semen. Semen is a vehicle for sexual transmission and saliva can be a vehicle of transmission through bites; other types of exposure, e.g., to saliva through kissing, are unlikely modes of transmission. Transmission of HBV via tears, sweat, urine, stool, or droplet nuclei has not been clearly documented.
In the United States, the most important routes of transmission are perinatal and sexual contact, either heterosexual or homosexual, with an infected person. Fecal-oral transmission does not appear to occur. However, transmission occurs among men who have sex with men, possibly via contamination from asymptomatic rectal mucosal lesions. In the past two decades, outbreaks of hepatitis B have occurred in long-term care facilities (e.g., assisted living facilities and nursing homes) as the result of lack of infection control practices related to blood glucose monitoring.
Hepatitis B Perinatal Transmission*
- If mother positive for HBsAg and HBeAg
- 70%-90% of infants infected
- 90% of infected infants become chronically infected
- If positive for HBsAg only
- 10% of infants infected
- 90% of infected infants become chronically infected
*in the absence of postexposure prophylaxis
Hepatitis B virus remains infectious for at least 7 days on environmental surfaces and is transmissible in the absence of visible blood. Direct percutaneous inoculation of HBV by needles during injection-drug use is an important mode of transmission. Breaks in the skin without overt needle puncture, such as fresh cutaneous scratches, abrasions, burns, or other lesions, may also serve as routes for entry. Nosocomial exposures such as transfusion of blood or blood products, hemodialysis, use of meters and lancets for glucose monitoring, insulin pens, and needle-stick or other “sharps” injuries sustained by hospital personnel have all resulted in HBV transmission. Rare transmission to patients from HBsAg-positive health care personnel has been documented. Outbreaks have been reported among patients in dialysis centers in many countries through failure to adhere to recommended infection control practices against transmission of HBV and other blood-borne pathogens in these settings. IG, heat-treated plasma protein fraction and albumin are considered safe. In the past, outbreaks have been traced to tattoo parlors, acupuncturists, and barbers.
Contamination of mucosal surfaces with infective serum or plasma may occur during mouth pipetting, eye splashes, or other direct contact with mucous membranes of the eyes or mouth, such as hand-to-mouth or hand-to-eye contact when hands are contaminated with infective blood or serum. Transfer of infective material to skin lesions or mucous membranes via inanimate environmental surfaces may occur by touching surfaces of various types of hospital equipment. Contamination of mucosal surfaces with infective secretions other than serum or plasma could occur with contact involving semen.
Perinatal transmission from mother to infant at birth is very efficient. If the mother is positive for both HBsAg and HBeAg, 70%–90% of infants will become infected in the absence of postexposure prophylaxis. The risk of perinatal transmission is about 10% if the mother is positive only for HBsAg. As many as 90% of infant HBV infections will progress to chronic infection.
The frequency of infection and patterns of transmission vary in different parts of the world. Approximately 45% of the global population live in areas with a high prevalence of chronic HBV infection (8% or more of the population is HBsAg positive), 43% in areas with a moderate prevalence (2% to 7% of the population is HBsAg positive), and 12% in areas with a low prevalence (less than 2% of the population is HBsAg positive).
Global Patterns of Chronic HBV Infection
- High (>8%): 45% of global population
- lifetime risk of infection >60%
- early childhood infections common
- Intermediate (2%-7%): 43% of global population
- lifetime risk of infection 20%-60%
- infections occur in all age groups
- Low (<2%): 12% of global population
- lifetime risk of infection <20%
- most infections occur in adult risk groups
In China, Southeast Asia, most of Africa, most Pacific Islands, parts of the Middle East, and the Amazon Basin, 8% to l5% of the population carry the virus. The lifetime risk of HBV infection is greater than 60%, and most infections are acquired at birth or during early childhood, when the risk of developing chronic infections is greatest. In these areas, because most infections are asymptomatic, very little acute disease related to HBV occurs, but rates of chronic liver disease and liver cancer among adults are very high. In the United States, Western Europe, and Australia, HBV infection is a disease of low endemicity. Infection occurs primarily during adulthood, and only 0.1% to 0.5% of the population are chronic carriers. Lifetime risk of HBV infection is less than 20% in low prevalence areas.
Communicability
Persons with either acute or chronic HBV infection should be considered infectious any time that HBsAg is present in the blood. When symptoms are present in persons with acute HBV infection, HBsAg can be found in blood and body fluids for 1–2 months before and after the onset of symptoms.
Hepatitis B — United States, 1978-2012
Age of Infection of Acute and Chronic Hepatitis B Virus Infection
CDC Sentinel Sites. 1989 data.
Risk Factors for Hepatitis B
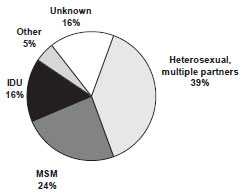
MMWR 2006;55(RR-16):6-7
Secular Trends in the United States
Hepatitis has been reportable in the United States for many years. Hepatitis B became reportable as a distinct entity during the 1970s, after serologic tests to differentiate different types of hepatitis became widely available.
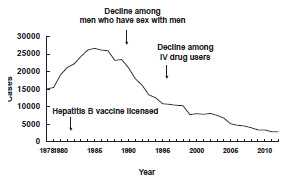
The incidence of reported hepatitis B peaked in the mid-1980s, with about 26,000 cases reported each year. Reported cases have declined since that time, and fell below 10,000 cases for the first time in 1996. The decline in cases during the 1980s and early 1990s is generally attributed to reduction of transmission among men who have sex with men and injection-drug users, as a result of HIV prevention efforts.
During 1990–2004, incidence of acute hepatitis B in the United States declined 75%. The greatest decline (94%) occurred among children and adolescents, coincident with an increase in hepatitis B vaccine coverage. A total of 2,895 cases of hepatitis B were reported in 2012.
An estimated 800,000 to 1.4 million persons in the United States are chronically infected with HBV, and an additional 5,000–8,000 persons become chronically infected each year.
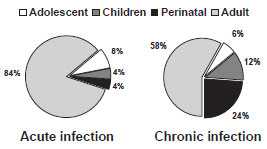
Before routine childhood hepatitis B vaccination was recommended, more than 80% of acute HBV infections occurred among adults. Adolescents accounted for approximately 8% of infections, and children and infants infected through perinatal transmission accounted for approximately 4% each. Perinatal transmission accounted for a disproportionate 24% of chronic infections.
In the United States in 2005, the highest incidence of acute hepatitis B was among adults aged 25–45 years. Approximately 79% of persons with newly acquired hepatitis B infection are known to engage in high-risk sexual activity or injection-drug use. Other known exposures (e.g., occupational, household, travel, and healthcare-related) together account for 5% of new infections. Approximately 16% of persons deny a specific risk factor for infection.
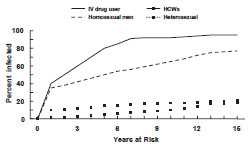
Although HBV infection is uncommon among adults in the general population (the lifetime risk of infection is less than 20%), it is highly prevalent in certain groups. Risk for infection varies with occupation, lifestyle, or environment. Generally, the highest risk for HBV infection is associated with lifestyles, occupations, or environments in which contact with blood from infected persons is frequent. In addition, the prevalence of HBV markers for acute or chronic infection increases with increasing number of years of high-risk behavior. For instance, an estimated 40% of injection-drug users become infected with HBV after 1 year of drug use, while more than 80% are infected after 10 years.
Hepatitis B Virus Infection by Duration of High-Risk Behavior
Strategy to Eliminate Hepatitis B Virus Transmission—United States
- Prevent perinatal HBV transmission
- Routine vaccination of all infants
- Vaccination of children in high-risk groups
- Vaccination of adolescents
- Vaccination of adults in high-risk groups
Hepatitis B Prevention Strategies
Hepatitis B vaccines have been available in the United States since 1981. Vaccines have had a large impact on acute Hepatitis B disease. However, the impact of vaccine on chronic HBV disease has been less than optimal. However there are examples of positive effects, such as dramatic reductions in complications of hepatocellular carcinoma observed in Alaska Natives.
The three major risk groups (heterosexuals with multiple partners or contact with infected persons, injection-drug users, and men who have sex with men) are not reached effectively by targeted programs. Deterrents to immunization of these groups include lack of awareness of the risk of disease and its consequences, lack of effective public or private sector programs, and vaccine cost. Difficulty in gaining access to these populations is also a problem.
A comprehensive strategy to eliminate hepatitis B virus transmission was recommended in 1991; it includes prenatal testing of pregnant women for HBsAg to identify newborns who require immunoprophylaxis for prevention of perinatal infection and to identify household contacts who should be vaccinated, routine vaccination of infants, vaccination of adolescents, and vaccination of adults at high risk for infection. Recommendations to further enhance vaccination of adults at increased risk of HBV infection were published in 2011.
Hepatitis B Vaccine
Hepatitis B Vaccine
- Composition
- recombinant HBsAg
- Efficacy
- 95% (Range, 80%-100%)
- Duration of Immunity
- 20 years or more
- Schedule
- 3 Doses
- Booster doses not routinely recommended
Hepatitis B Vaccine Formulations
-
Recombivax HB (Merck)
- 5 mcg/0.5 mL (pediatric)
- 10 mcg/1 mL (adult)
- 40 mcg/1 mL (dialysis) -
Engerix-B (GSK)
- 10 mcg/0.5 mL (pediatric)
- 20 mcg/1 mL (adult)
Characteristics
A plasma-derived vaccine was licensed in the United States in 1981. It was produced from 22-nm HBsAg particles purified from the plasma of chronically infected humans. The vaccine was safe and effective but was not well accepted, possibly because of unsubstantiated fears of transmission of live HBV and other bloodborne pathogens (e.g., human immunodeficiency virus). This vaccine was removed from the U.S. market in 1992.
The first recombinant hepatitis B vaccine was licensed in the United States in July 1986. A second, similar vaccine was licensed in August 1989.
Recombinant vaccine is produced by inserting a plasmid containing the gene for HBsAg into common baker’s yeast (Saccharomyces cerevisiae). Yeast cells then produce HBsAg, which is harvested and purified. The recombinant vaccine contains more than 95% HBsAg protein (5 to 40 mcg/mL); yeast-derived proteins may constitute up to 5% of the final product, but no yeast DNA is detectable in the vaccine. HBV infection cannot result from use of the recombinant vaccine, since no potentially infectious viral DNA or complete viral particles are produced in the recombinant system. Vaccine HBsAg is adsorbed to aluminum hydroxide or aluminum hydroxyphosphate sulfate.
Hepatitis B vaccine is produced by two manufacturers in the United States, Merck (Recombivax HB) and GlaxoSmithKline Pharmaceuticals (Engerix-B). Both vaccines are available in both pediatric and adult formulations. Although their antigen content differs, the two vaccines are interchangeable, except for the two-dose schedule for adolescents aged 11 through 15 years. Only Merck vaccine is approved for this schedule. Providers must always follow the manufacturer’s dosage recommendations, which may vary by product.
Both the pediatric and adult formulations of Recombivax HB are approved for use in any age group. For example, the adult formulation of Recombivax HB may be used in children (0.5 mL) and adolescents (0.5 mL). However, pediatric Engerix-B is approved for use only in children and adolescents younger than 20 years of age. The adult formulation of Engerix-B is not approved for use in infants and children but may be used in both adolescents (11 through 19 years of age) and adults.
Engerix-B contains aluminum hydroxide as an adjuvant, and Recombivax HB contains aluminum hydroxyphosphate sulfate as an adjuvant. Both vaccines are supplied in single-dose vials and syringes, and no formulation of either vaccine contains thimerosal or any other preservative.
Immunogenicity and Vaccine Efficacy
After three intramuscular doses of hepatitis B vaccine, more than 90% of healthy adults and more than 95% of infants, children, and adolescents (from birth to 19 years of age) develop adequate antibody responses. However, there is an age-specific decline in immunogenicity. After age 40 years, approximately 90% of recipients respond to a three-dose series, and by 60 years, only 75% of vaccinees develop protective antibody titers. The proportion of recipients who respond to each dose varies by age.
The vaccine is 80% to 100% effective in preventing infection or clinical hepatitis in those who receive the complete vaccine series. Larger vaccine doses (2 to 4 times the normal adult dose), or an increased number of doses, are required to induce protective antibody in most hemodialysis patients and may also be necessary for other immunocompromised persons.
The recommended dosage of vaccine differs depending on the age of the recipient and type of vaccine (see table). Hemodialysis patients should receive a 40-mcg dose in a series of three or four doses. Recombivax HB has a special dialysis patient formulation that contains 40 mcg/mL.
The deltoid muscle is the recommended site for hepatitis B vaccination in adults and children, while the anterolateral thigh is recommended for infants and neonates. Immunogenicity of vaccine in adults is lower when injections are given in the gluteus. Hepatitis B vaccine should be administered to newborns using a needle of at least 5/8 inch length and to older children and adults of at least 1 inch length. Hepatitis B vaccine administered by any route or site other than intramuscularly in the anterolateral thigh or deltoid muscle should not be counted as valid and should be repeated unless serologic testing indicates that an adequate response has been achieved.
| Age Group | Single-Antigen Vaccine | Combination Vaccine | ||||||
|---|---|---|---|---|---|---|---|---|
| Recombivax HB | Engerix-B | Pediarix | Twinrix | |||||
| Dose (mcg)* |
Volume(mL) | Dose (mcg)* |
Volume(mL) | Dose (mcg)* |
Volume(mL) | Dose (mcg)* |
Volume(mL) | |
| Infants (<1 year) | 5 | 0.5 | 10 | 0.5 | 10 | 0.5 | N/A | N/A |
| Children (1-10 years) | 5 | 0.5 | 10 | 0.5 | 10 | 0.5 | N/A | N/A |
| Adolescents (11-15 yrs) | 10† | 1.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Adolescents (11-19 yrs) | 5 | 0.5 | 10 | 0.5 | N/A | N/A | N/A | N/A |
| Adults (>20 years) | 10 | 1.0 | 20 | 1.0 | N/A | N/A | 20 | 1.0 |
| Hemodialysis patients and other immunocompromised persons (<20 yrs)§ | 5 | 0.5 | 10 | N/A | N/A | N/A | N/A | N/A |
| Hemodialysis patients and other immunocompromised persons (>20 yrs) | 40¶ | 1.0 | 40‡ | N/A | N/A | N/A | N/A | N/A |
* Recombinant hepatitis B surface antigen protein dose.
† Adult formulation administered on a 2-dose schedule.
§ Higher doses might be more immunogenic, but no specific recommendations have been made.
¶ Dialysis formulation administered on a 3-dose schedule at 0, 1, and 6 months.
‡ Two 1.0 mL doses administered at one site, on a 4-dose schedule at 0, 1, 2, and 6 months.
** Not applicable.
Hepatitis B Vaccine Long-term Efficacy
- Immunologic memory established following vaccination
- Exposure to HBV results in anamnestic anti-HBs response
- Chronic infection rarely documented among vaccine responders
Hepatitis B Vaccine
- Routine booster doses are NOT routinely recommended for any group
Available data show that vaccine-induced antibody levels decline with time. However, immune memory remains intact for more than 20 years following immunization, and both adults and children with declining antibody levels are still protected against significant HBV infection (i.e., clinical disease, HBsAg antigenemia, or significant elevation of liver enzymes). Exposure to HBV results in an anamnestic anti-HBs response that prevents clinically significant HBV infection. Chronic HBV infection has only rarely been documented among vaccine responders.
For adults and children with normal immune status, booster doses of vaccine are not recommended. Routine serologic testing to assess immune status of vaccinees is not recommended. The need for booster doses after longer intervals will continue to be assessed as additional information becomes available.
For hemodialysis patients, the need for booster doses should be assessed by annual testing of vaccinees for antibody levels, and booster doses should be provided when antibody levels decline below 10 mIU/mL.
Hepatitis B Vaccine Routine Infant Schedule
| Dose | Usual Age | Minimum Interval |
|---|---|---|
| Primary 1 | Birth | --- |
| Primary 2 | 1-2 months | 4 weeks |
| Primary 3 | 6-18 months* | 8 weeks** |
* infants who mothers are HBsAg+ or whose HBsAg status is unknown should receive the third dose at 6 months of age
** at least 16 weeks after the first dose
Third Dose of Hepatitis B Vaccine
- Minimum of 8 weeks after second dose, and
- At least 16 weeks after first dose, and
- For infants, at least 24 weeks of age
Preterm Infants
- Birth dose and HBIG if mother HBsAg positive (within 12 hours of birth)
- Preterm infants who weigh less than 2,000 grams have a decreased response to vaccine administered before 1 month of age
- Delay first dose until chronologic age 1 month if mother documented to be HBsAg negative at the time of birth
Vaccination Schedule and Use
Infants and Children
Hepatitis B vaccination is recommended for all infants soon after birth and before hospital discharge. Infants and children younger than 11 years of age should receive 0.5 mL (5 mcg) of pediatric or adult formulation Recombivax HB (Merck) or 0.5 mL (10 mcg) of pediatric Engerix-B (GlaxoSmithKline). Primary vaccination consists of three intramuscular doses of vaccine. The usual schedule is 0, 1 to 2, and 6 to 18 months. Infants whose mothers are HBsAg positive or whose HBsAg status is unknown should receive the last dose by 6 months of age (12 to 15 months if Comvax is used).
Because the highest titers of anti-HBs are achieved when the last two doses of vaccine are spaced at least 4 months apart, schedules that achieve this spacing are preferable. However, schedules with 2-month intervals between doses, which conform to schedules for other childhood vaccines, have been shown to produce good antibody responses and may be appropriate in populations in which it is difficult to ensure that infants will be brought back for all their vaccinations. However, the third dose must be administered at least 8 weeks after the second dose, and at least 16 weeks after the first dose. For infants, the third dose should not be given earlier than 24 weeks of age. It is not necessary to add doses or restart the series if the interval between doses is longer than recommended.
Preterm infants born to HBsAg-positive women and women with unknown HBsAg status must receive immunoprophylaxis with hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth. See the section on Postexposure Management for additional information. Preterm infants weighing less than 2,000 grams have a decreased response to hepatitis B vaccine administered before 1 month of age. However, by chronologic age 1 month, preterm infants, regardless of initial birthweight or gestational age, are as likely to respond as adequately as full-term infants. Preterm infants of low birthweight whose mothers are HBsAg negative can receive the first dose of the hepatitis B vaccine series at chronologic age 1 month. Preterm infants discharged from the hospital before chronologic age 1 month can receive hepatitis B vaccine at discharge if they are medically stable and have gained weight consistently. The full recommended dose should be used. Divided or reduced doses are not recommended.
Comvax
COMVAX
- Hepatitis B-Hib combination
- Use when either antigen is indicated
- Cannot use at younger than 6 weeks of age
- May be used in infants whose mother is HBsAg positive or status is unknown
Hepatitis B vaccine is available in combination with Haemophilus influenzae type b (Hib) vaccine as Comvax (Merck). Each dose of Comvax contains 7.5 mcg of PRP-OMP Hib vaccine (PedvaxHIB), and 5 mcg of hepatitis B surface antigen. The dose of hepatitis B surface antigen is the same as that contained in Merck’s pediatric formulation. The immunogenicity of the combination vaccine is equivalent to that of the individual antigens administered at separate sites.
Comvax is licensed for use at 2, 4, and 12 through 15 months of age. It may be used whenever either antigen is indicated and the other antigen is not contraindicated. However, the vaccine must not be administered to infants younger than 6 weeks of age because of potential suppression of the immune response to the Hib component (see Chapter 8, Haemophilus influenzae type b, for more details). Although it is not labeled for this indication by FDA, ACIP recommends that Comvax may be used in infants whose mothers are HBsAg positive or whose HBsAg status is unknown. Comvax will be removed from existing contracts and pricing programs in early 2015.
Pediarix
Pediarix
- DTaP – Hep B – IPV combination
- Approved for 3 doses at 2, 4 and 6 months
- Not approved for booster doses
- Approved for children 6 weeks to 7 years of age
- May be used interchangeably with other pertussis-containing vaccines if necessary
- Can be given at 2, 4, and 6 months to infants who received a birth dose of hepatitis B vaccine (total of 4 doses)
- May be used in infants whose mothers are HBsAg positive or status unknown
In 2002, the Food and Drug Administration approved Pediarix (GlaxoSmithKline), the first pentavalent (5-component) combination vaccine licensed in the United States. Pediarix contains DTaP (Infanrix), hepatitis B (Engerix-B), and inactivated polio vaccines. In prelicensure studies, children who received these vaccine antigens together as Pediarix were at least as likely to develop a protective level of antibody as those who received the vaccines separately; and their antibody titers were also at least as high.
The minimum age for the first dose of Pediarix is 6 weeks, so it cannot be used for the birth dose of the hepatitis B series. Pediarix is approved for the first three doses of the DTaP and IPV series, which are usually given at about 2, 4, and 6 months of age; it is not approved for fourth or fifth (booster) doses of the DTaP or IPV series. However, Pediarix is approved for use through 6 years of age. A child who is behind schedule can still receive Pediarix as long as it is given for doses 1, 2, or 3 of the series, and the child is younger than 7 years of age.
A dose of Pediarix inadvertently administered as the fourth or fifth dose of the DTaP or IPV series does not need to be repeated.
Pediarix may be used interchangeably with other pertussis-containing vaccines if necessary (although ACIP prefers the use of the same brand of DTaP for all doses of the series, if possible). It can be given at 2, 4, and 6 months to infants who received a birth dose of hepatitis B vaccine (total of 4 doses of hepatitis B vaccine). Although not labeled for this indication by FDA, Pediarix may be used in infants whose mothers are HBsAg positive or whose HBsAg status is unknown.
Adolescents
Hepatitis B Vaccine Adolescent Vaccination
- Routine vaccination recommended through age 18 years
- Integrate into routine adolescent immunization visit
- Flexible schedules
Hepatitis B Vaccine Adolescent and Adult Schedule
| Dose | Usual Interval | Minimum Interval |
|---|---|---|
| Primary 1 | --- | --- |
| Primary 2 | 1 month | 4 weeks |
| Primary 3 | 5 months | 8 weeks* |
*third dose must be separated from first dose by at least 16 weeks
Alternative Adolescent Vaccination Schedule
- Two 1.0 mL (10 mcg) doses of Recombivax HB separated by 4-6 months
- Approved only for adolescents 11-15 years of age
- Only applies to Merck hepatitis B vaccine
Routine hepatitis B vaccination is recommended for all children and adolescents through age 18 years. All children not previously vaccinated with hepatitis B vaccine should be vaccinated at 11 or 12 years of age with the age-appropriate dose of vaccine. When adolescent vaccination programs are being considered, local data should be considered to determine the ideal age group (e.g., preadolescents, young adolescents) to vaccinate to achieve the highest vaccination rates. The vaccination schedule should be flexible and should take into account the feasibility of delivering three doses of vaccine to this age group. Unvaccinated older adolescents should be vaccinated whenever possible. Those in groups at risk for HBV infection (e.g., Asian and Pacific Islanders, sexually active) should be identified and vaccinated in settings serving this age group (i.e., schools, sexually transmitted disease clinics, detention facilities, drug treatment centers).
Persons younger than 20 years of age should receive 0.5 mL (5 mcg) of pediatric or adult formulation Recombivax HB (Merck) or 0.5 mL (10 mcg) of pediatric formulation Engerix-B (GlaxoSmithKline). The adult formulation of Engerix-B may be used in adolescents, but the approved dose is 1 mL (20 mcg).
The usual schedule for adolescents is two doses separated by no less than 4 weeks, and a third dose 4 to 6 months after the second dose. If an accelerated schedule is needed, the minimum interval between the first two doses is 4 weeks, and the minimum interval between the second and third doses is 8 weeks. However, the first and third doses should be separated by no less than 16 weeks. Doses given at less than these minimum intervals should not be counted and should be repeated.
In 1999, the Food and Drug Administration approved an alternative hepatitis B vaccination schedule for adolescents 11 through 15 years of age. This alternative schedule is for two 1.0-mL (10 mcg) doses of Recombivax HB separated by 4 to 6 months. Seroconversion rates and postvaccination anti-HBs antibody titers were similar using this schedule or the standard schedule of three 5-mcg doses of Recombivax HB. This alternative schedule is approved only for adolescents 11 through 15 years of age, and for Merck’s hepatitis B vaccine. The 2-dose schedule should be completed by the 16th birthday.
Adults (20 Years of Age and Older)
Adults at Risk for HBV Infection
- Sexual exposure
- sex partners of HBsAg-positive persons
- sexually active persons not in a long-term, mutually monogamous relationship*
- persons seeking evaluation or treatment for a sexually transmitted disease
- men who have sex with men
- Percutaneous or mucosal exposure to blood
- current or recent IDU
- household contacts of HBsAg-positive persons
- residents and staff of facilities for developmentally disabled persons
- healthcare and public safety workers with risk for exposure to blood or blood-contaminated body fluids
- persons with end-stage renal disease
- persons with diabetes mellitus
- Other groups
- international travelers to regions with high or intermediate levels (HBsAg prevalence of 2% or higher) of endemic HBV infection
- persons with HIV infection
*persons with more than one sex partner during the previous 6 months
Routine preexposure vaccination should be considered for adults who are at increased risk of HBV infection. Adults 20 years of age and older should receive 1 mL (10 mcg) of pediatric or adult formulation Recombivax HB (Merck) or 1 mL (20 mcg) of adult formulation Engerix-B (GlaxoSmithKline). The pediatric formulation of Engerix-B is not approved for use in adults.
The usual schedule for adults is two doses separated by no less than 4 weeks, and a third dose 4 to 6 months after the second dose. If an accelerated schedule is needed, the minimum interval between the first two doses is 4 weeks, and the minimum interval between the second and third doses is 8 weeks. However, the first and third doses should be separated by no less than 16 weeks. Doses given at less than these minimum intervals should not be counted and should be repeated. It is not necessary to restart the series or add doses because of an extended interval between doses.
Hepatitis B vaccination is recommended for all unvaccinated adults at risk for HBV infection and for all adults requesting protection from HBV infection. Acknowledgment of a specific risk factor should not be a requirement for vaccination.
Persons at risk for infection by sexual exposure include sex partners of HBsAg-positive persons, sexually active persons who are not in a long-term, mutually monogamous relationship (e.g., persons with more than one sex partner during the previous 6 months), persons seeking evaluation or treatment for a sexually transmitted disease, and men who have sex with men.
Persons at risk for infection by percutaneous or mucosal exposure to blood include current or recent injection-drug users (IDU), household contacts of HBsAg-positive persons, residents and staff of facilities for developmentally disabled persons, healthcare and public safety workers with risk for exposure to blood or blood-contaminated body fluids, and persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients.
Adults with diabetes mellitus (type 1 or type 2) are at increased risk of HBV infection, probably because of breaches in infection control during assisted blood glucose monitoring (e.g., reuse of single patient finger stick devices). In October 2011, ACIP recommended that all previously unvaccinated adults 19 through 59 years of age with diabetes mellitus type 1 and type 2 be vaccinated against hepatitis B as soon as possible after a diagnosis of diabetes is made. ACIP also recommends that unvaccinated adults 60 years of age or older with diabetes may be vaccinated at the discretion of the treating clinician after assessing their risk and the likelihood of an adequate immune response to vaccination.
Other groups at risk include international travelers to regions with high or intermediate levels (HBsAg prevalence of 2% or higher) of endemic HBV infection, long term travelers, and those who may engage in high-risk behaviors or provide health-care while traveling. Persons with HIV infection are also at increased risk.
In settings in which a high proportion of adults have risks for HBV infection (e.g., sexually transmitted disease/human immunodeficiency virus testing and treatment facilities, drug-abuse treatment and prevention settings, healthcare settings targeting services to IDUs, healthcare settings targeting services to MSM, and correctional facilities), ACIP recommends hepatitis B vaccination for all unvaccinated adults. In other primary care and specialty medical settings in which adults at risk for HBV infection receive care, healthcare providers should inform all patients about the health benefits of vaccination, risks for HBV infection, and persons for whom vaccination is recommended; and should vaccinate any adults who report risks for HBV infection or request protection from HBV infection.
Twinrix
In 2001, the Food and Drug Administration approved a combination hepatitis A and hepatitis B vaccine (Twinrix, GlaxoSmithKline). Each dose of Twinrix contains 720 ELISA units of hepatitis A vaccine (equivalent to a pediatric dose of Havrix), and 20 mcg of hepatitis B surface antigen protein (equivalent to an adult dose of Engerix-B). The vaccine is administered in a three-dose series at 0, 1, and 6 months. Appropriate spacing of the doses must be maintained to assure long-term protection from both vaccines. The first and third doses of Twinrix should be separated by at least 6 months. The first and second doses should be separated by at least 4 weeks, and the second and third doses should be separated by at least 5 months. In 2007, the FDA approved an alternative Twinrix schedule of doses at 0, 7, and 21–31 days and a booster dose 12 months after the first dose. It is not necessary to restart the series or add doses if the interval between doses is longer than the recommended interval.
Twinrix is approved for persons 18 years of age and older, and can be administered to persons in this age group for whom either hepatitis A and hepatitis B vaccines is recommended. Because the hepatitis B component of Twinrix is equivalent to a standard adult dose of hepatitis B vaccine, the schedule is the same whether Twinrix or single-antigen hepatitis B vaccine is used. Single-antigen hepatitis A vaccine can be used to complete a series begun with Twinrix or vice versa. See the Hepatitis A chapter for details.
Serologic Testing of Vaccine Recipients
Prevaccination Serologic Testing
- Not indicated before routine vaccination of infants or children
- Recommended for
- all persons born in Africa, Asia, the Pacific Islands, and other regions with HBsAg prevalence of 2% or higher
- household, sex, and needle-sharing contacts of HBsAg-positive persons
- men who have sex with men
- injection drug users
- certain persons receiving cytotoxic or immunosuppressive therapy
Prevaccination Serologic Testing
The decision to screen potential vaccine recipients for prior infection depends on the cost of vaccination, the cost of testing for susceptibility, and the expected prevalence of immune persons in the population being screened. Prevaccination testing is recommended for all foreign-born persons (including immigrants, refugees, asylum seekers, and internationally adopted children) born in Africa, Asia, the Pacific Islands, and other regions with endemicity of HBV infection; household, sex, and needle-sharing contacts of HBsAg-positive persons; men who have sex with men; injection drug users; and certain persons receiving cytotoxic or immunosuppressive therapy. Screening is usually cost-effective, and should be considered for groups with a high risk of HBV infection (prevalence of HBV markers 20% or higher), such as men who have sex with men, injection-drug users, and incarcerated persons. Screening is usually not cost-effective for groups with a low expected prevalence of HBV serologic markers, such as health professionals in their training years.
Serologic testing is not recommended before routine vaccination of infants, children, or adolescents.
Postvaccination Serologic Testing
Postvaccination Serologic Testing
- Not routinely recommended following vaccination of infants, children, adolescents, or most adults
- Recommended for
- chronic hemodialysis patients
- other immunocompromised persons
- persons with HIV infection
- sex partners of HBsAg+ persons
- infants born to HBsAg+ women
- certain healthcare personnel
- healthcare personnel who have contact with patients or blood should be tested for anti-HBs (antibody to hepatitis B surface antigen) 1 to 2 months after completion of the 3-dose series
Testing for immunity following vaccination is not recommended routinely but should be considered for persons whose subsequent management depends on knowledge of their immune status, such as chronic hemodialysis patients, other immunocompromised persons, and persons with HIV infection. Testing is also recommended for sex partners of HBsAg-positive persons. Postvaccination testing should be performed 1 to 2 months after completion of the vaccine series.
Infants born to HBsAg-positive women should be tested for HBsAg and antibody to HBsAg (anti-HBs) 1 to 2 months after completion of the final dose of the hepatitis B vaccine series, and at least age 9 months (generally at the next well-child visit). If HBsAg is not present and anti-HBs antibody is present, children can be considered to be protected.
Healthcare personnel who have contact with blood and body fluids of patients who might be infected with HBV, or who are at ongoing risk for injuries with sharp instruments or needlesticks should be routinely tested for antibody 1 to 2 months after completion of the 3-dose hepatitis B vaccine series, assuming they are not previously vaccinated. Data since 2002 indicate the rates of reported exposures are highest among healthcare trainees, and vary by occupation and job duties among non-trainee healthcare personnel (e.g., low for office-based counseling, higher for healthcare personnel performing procedures). All health-care institutions should ensure healthcare personnel receive training to recognize and report exposures, have systems in place to facilitate reporting and postexposure assessment, and have prophylaxis readily accessible for timely administration.
Increasingly, healthcare personnel with documentation of routine hepatitis B vaccination received the series in infancy or as catch-up vaccination in adolescence without postvaccination testing. Antibody to vaccine antigen wanes over time, although protection persists in vaccine recipients who responded initially. A negative anti-HBs serologic response in healthcare personnel who received hepatitis B vaccine in the distant past will not distinguish between failure to respond to the initial vaccination series (lack of protection) and response to the initial vaccination series with subsequent waning of antibody (protected).
CDC recommends evaluating healthcare personnel for hepatitis B virus protection either at matriculation or hire (preexposure) or with post-exposure management, depending on the occupational risk for exposure to potentially contaminated blood or body fluids, and the prevalence of hepatitis B infection in the patient population. Booster doses of hepatitis B vaccine are not recommended for persons with normal immune systems. However, previously vaccinated healthcare personnel for whom preexposure evaluation fails to detect protective anti-HBs should receive a “challenge dose” of hepatitis B vaccine to assess protection, which will be indicated by a rise in anti-HBs, or “memory” response to vaccine antigen.
Healthcare personnel who respond to the challenge dose do not require additional management, even if exposed. Healthcare personnel who do not respond to a challenge dose should complete revaccination and retesting for anti-HBs. Postexposure management of healthcare personnel ensures hepatitis B prophylaxis and assesses vaccine response as dictated by the HBsAg status of the source patient. Detailed guidance for pre- or postexposure evaluation and management of healthcare personnel for hepatitis B protection was published in 2013.
Vaccine Nonresponse
Management of Nonresponse to Hepatitis B Vaccine
- Complete a second series of three doses
- Should be given on the usual schedule of 0, 1 and 6 months (may be given on a 0, 1, and 4 month or a 0, 2 and 4 month schedule)
- Retest 1-2 months after completing the second series
Persistent Nonresponse to Hepatitis B Vaccine
- Less than 5% of vaccinees do not develop anti-HBs after 6 valid doses
- May be nonresponder or “hyporesponder”
- Check HBsAg status
- If exposed, treat as nonresponder with postexposure prophylaxis
Several factors have been associated with nonresponse to hepatitis B vaccine. These include vaccine factors (e.g., dose, schedule, injection site) and host factors. Older age (40 years and older), male sex, obesity, smoking, and chronic illness have been independently associated with nonresponse to hepatitis B vaccine. Additional vaccine doses for persons who receive post-vaccination testing and who fail to respond to a primary vaccination series administered in the deltoid muscle produce adequate response in 15% to 25% of vaccinees after one additional dose and in 30% to 50% after three additional doses.
Persons who do not respond to the first series of hepatitis B vaccine should complete a second three-dose vaccine series. The second vaccine series should be given on the usual 0, 1, 6-month schedule. Healthcare personnel and others for whom postvaccination serologic testing is recommended should be retested 1 to 2 months after completion of the second vaccine series.
Fewer than 5% of persons receiving six doses of hepatitis B vaccine administered by the appropriate schedule in the deltoid muscle fail to develop detectable anti-HBs antibody. One reason for persistent nonresponse to hepatitis B vaccine is chronic infection with HBV. Persons who fail to develop detectable anti-HBs after six doses should be tested for HBsAg. Persons who are found to be HBsAg positive should be counseled accordingly. Persons who fail to respond to two appropriately administered three-dose series, and who are HBsAg negative should be considered susceptible to HBV infection and should be counseled regarding precautions to prevent HBV infection and the need to obtain HBIG prophylaxis for any known or probable parenteral exposure to HBsAg-positive blood (see the postexposure prophylaxis table in this chapter).
It is difficult to interpret a negative anti-HBs serologic response in a person who received hepatitis B vaccine in the past and was not tested after vaccination. Without postvaccination testing 1 to 2 months after completion of the series, it is not possible to determine if persons testing negative years after vaccination represent true vaccine failure (i.e., no initial response), or have anti-HBs antibody that has waned to below a level detectable by the test. The latter is the most likely explanation, because up to 60% of vaccinated people lose detectable antibody (but not protection) 9 to 15 years after vaccination.
Postexposure Management
After a percutaneous (needle stick, laceration, bite) or permucosal exposure that contains or might contain HBV, blood should be obtained from the person who was the source of the exposure to determine their HBsAg status. Management of the exposed person depends on the HBsAg status of the source and the vaccination and anti-HBs response status of the exposed person. Recommended postexposure prophylaxis is described in the following table.
| Vaccination and antibody response status of exposed person |
Treatment | ||
|---|---|---|---|
| Source HBsAg-positive | Source HBsAg-negative | Source not tested or status unknown | |
| Unvaccinated | HBIG x 1; Initiate HB vaccine series | Initiate HB vaccine series | Initiate HB vaccine series |
| Previously vaccinated - known responder | No treatment | No treatment | No treatment |
| Previously vaccinated - known nonresponder after 3 doses | HBIG x 1 and initiate revaccination | No treatment | If known high-risk source, treat as if source were HBsAg-positive. |
| Previously vaccinated - known nonresponder after 6 doses | HBIG x 2 (separated by 1 month) | No treatment | If known high-risk source,treat as if source were HBsAg-positive. |
| Antibody response unknown | Test exposed person for anti-HBs - If adequate,* no treatment, - If inadequate,* HBIG x 1 and vaccine booster | No treatment | Test exposed person for anti-HBs - If adequate,* no treatment - If inadequate,* HBIG x 1 and vaccine booster |
Abbreviations: HbsAg = hepatitis B surface antigen; HBIG = hepatitis B immune globulin; anti-HBs = antibody to hepatitis B surface antigen;
HB = hepatitis B.
Source: Adapted from CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: Immunization of adults. MMWR 2006;55(No. RR-16).
* A seroprotective (adequate) level of anti-HBs after completion of a vaccination series is defined as anti-HBs >10 mIU/mL; a response <10 mIU/mL is inadequate and is not a reliable indicator of protection.
Source: MMWR 2011:60(RR-7)42.
Hepatitis B vaccine is recommended as part of the therapy used to prevent hepatitis B infection following exposure to HBV. Depending on the exposure circumstance, the hepatitis B vaccine series may be started at the same time as treatment with hepatitis B immune globulin (HBIG).
HBIG is prepared by cold ethanol fraction of plasma from selected donors with high anti-HBs titers; it contains an anti-HBs titer of at least 1:100,000, by RIA. It is used for passive immunization for accidental (percutaneous, mucous membrane) exposure, sexual exposure to an HBsAg-positive person, perinatal exposure of an infant, or household exposure of an infant younger than 12 months old to a primary caregiver with acute hepatitis B. Most candidates for HBIG are, by definition, in a high-risk category and should therefore also receive hepatitis B vaccine.
Immune globulin (IG) is prepared by cold ethanol fractionation of pooled plasma and contains low titers of anti-HBs. Because titers are relatively low, IG has no valid current use for HBV disease unless hepatitis B immune globulin is unavailable.
Infants born to women who are HBsAg-positive (i.e., acutely or chronically infected with HBV) are at extremely high risk of HBV transmission and chronic HBV infection. Hepatitis B vaccination and one dose of HBIG administered within 24 hours after birth are 85%–95% effective in preventing chronic HBV infection. Hepatitis B vaccine administered alone beginning within 24 hours after birth is 70%–95% effective in preventing perinatal HBV infection.
The first dose of hepatitis B vaccine and HBIG (0.5 mL) should be given intramuscularly (IM), and are recommended for administration within 12 hours of birth. The hepatitis B vaccine dose is given at the same time as HBIG, but at a different site. The second and third vaccine doses should be given 1 to 2 months and 6 months, respectively, after the first dose. To monitor the success of therapy, testing for HBsAg and anti-HBs is recommended 1-2 months after the final vaccine dose but not before 9 months of age. If the mother’s HBsAg status is not known at the time of birth, the hepatitis B vaccination of the infant should be initiated within 12 hours of birth.
Prevention of Perinatal Hepatitis B Virus Infection
- Begin treatment within 12 hours of birth
- Hepatitis B vaccine (first dose) and HBIG at different sites
- Complete vaccination series at 6 months of age
- Test for response after completion of at least 3 doses of the HepB series at 9 through 18 months of age (generally at the next well-child visit)
HBIG given at birth does not interfere with the immune response to hepatitis B vaccine or other vaccines administered at 2 months of age.
Infants born to HBsAg-positive women and who weigh less than 2,000 grams at birth should receive postexposure prophylaxis as described above. However, the initial vaccine dose (at birth) should not be counted. The next dose in the series should be administered when the infant is chronologic age 1 month, followed by a third dose 1 to 2 months after the second , and the fourth dose at 6 months of age. To monitor the success of postexposure prophylaxis, testing for HBsAg and anti-HBs is recommended 1-2 months after the final vaccine dose, but not before 9 months of age.
Women admitted for delivery whose HBsAg status is unknown should have blood drawn for testing. While test results are pending, the infant should receive the first dose of hepatitis B vaccine (without HBIG) within 12 hours of birth. If the mother is found to be HBsAg positive, the infant should receive HBIG as soon as possible but not later than 7 days of age. If the infant does not receive HBIG, it is important that the second dose of vaccine be administered at 1 or 2 months of age.
Infants with birth weight less than 2,000 grams whose mother’s HBsAg status is unknown should receive hepatitis B vaccine within 12 hours of birth. If the maternal HBsAg status cannot be determined within 12 hours of birth HBIG should also be administered. The immune response to hepatitis B vaccine is less reliable in infants weighing less than 2,000 grams. The vaccine dose administered at birth should not be counted as part of the series, and the infant should receive three additional doses beginning at age 1 month (total number of doses should be at least 4). The vaccine series should be completed by 6 months of age.
Non-Occupational Exposure to an HBsAg-Positive Source
Persons who have written documentation of a complete hepatitis B vaccine series and who did not receive postvaccination testing should receive a single vaccine booster dose. Persons who are in the process of being vaccinated but who have not completed the vaccine series should receive the appropriate dose of HBIG and should complete the vaccine series. Unvaccinated persons should receive both HBIG and a dose of hepatitis B vaccine as soon as possible after exposure (preferably within 24 hours) and complete the 3-dose hepatitis B vaccine series according to the appropriate schedule. Hepatitis B vaccine may be administered simultaneously with HBIG in a separate injection site.
Household, sex, and needle-sharing contacts of HBsAg-positive persons should be identified. Unvaccinated sex partners and household and needle-sharing contacts should be tested for susceptibility to HBV infection and should receive the first dose of hepatitis B vaccine immediately after collection of blood for serologic testing. Susceptible persons should complete the vaccine series using an age-appropriate vaccine dose and schedule. Persons who are not fully vaccinated should complete the vaccine series.
Non-Occupational Exposure to a Source with Unknown HBsAg Status
Persons with written documentation of a complete hepatitis B vaccine series require no further treatment. Persons who are not fully vaccinated should complete the vaccine series. Unvaccinated persons should receive the hepatitis B vaccine series with the first dose administered as soon as possible after exposure, preferably within 24 hours.
Contraindications and Precautions to Vaccination
Hepatitis B Vaccine Contraindications and Precautions
- Severe allergic reaction to a vaccine component or following a prior dose
- Moderate or severe acute illness
Hepatitis B vaccination is contraindicated for persons with a history of hypersensitivity to yeast or any other vaccine component. Despite a theoretic risk for allergic reaction to vaccination in persons with allergy to Saccharomyces cerevisiae (baker’s yeast), no evidence exists to document adverse reactions after vaccination of persons with a history of yeast allergy.
Persons with a history of serious adverse events (e.g. anaphylaxis) after receipt of hepatitis B vaccine should not receive additional doses. As with other vaccines, vaccination of persons with moderate or severe acute illness, with or without fever, should be deferred until illness resolves. Vaccination is not contraindicated in persons with a history of multiple sclerosis (MS), Guillain-Barré syndrome (GBS), autoimmune disease (e.g. systemic lupus erythematosis or rheumatoid arthritis) or other chronic diseases.
Pregnancy is not a contraindication to vaccination. Limited data suggest that developing fetuses are not at risk for adverse events when hepatitis B vaccine is administered to pregnant women. Available vaccines contain non-infectious HBsAg and should cause no risk of infection to the fetus.
Adverse Events Following Vaccination
Hepatitis B Vaccine Adverse Reactions
- Anaphylaxis – one case per 1.1 million doses
Reported episodes of alopecia (hair loss) after rechallenge with hepatitis B vaccine suggest that vaccination might very rarely trigger episodes of alopecia. However, a population-based study found no statistically significant association between alopecia and hepatitis B vaccination.
In rare instances, other illnesses have been reported after hepatitis B vaccination, including GBS, chronic fatigue syndrome, neurologic disorders (e.g. leukoencephalitis, optic neuritis, and transverse myelitis), rheumatoid arthritis, type 1 diabetes, and autoimmune disease. However, no causal association between those conditions or any chronic illness and hepatitis B vaccine has been demonstrated. Reviews by scientific panels have also found no causal association between hepatitis B vaccination and MS.
Adverse Reactions Following Vaccination
Anaphylaxis has occurred after hepatitis B vaccination, with an estimated incidence of one case per 1.1 million vaccine doses distributed (95% confidence interval = 0.1 - 3.9) among children and adolescents.
Vaccine Storage and Handling
Hepatitis B vaccine should be maintained at refrigerator temperature between 35°F and 46°F (2°C and 8°C). Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
Acknowledgement
The editors thank Drs. Trudy Murphy, Phyllis Kozarsky, and Philip Spradling, CDC, for their assistance in updating this chapter.
Selected References
- Ascherio A, Zhang SM, Hernan MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med 2001;344:327–32.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: Immunization of infants, children, and adolescents. MMWR 2005;54(No. RR-16):1–32.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR 2006;55(No. RR-16):1–33.
- CDC. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR 2008;57(RR-8);9-11.
- CDC. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering posexposure management. MMWR 2013;62(RR-10): 1-19.
- CDC. Immunization of health-care personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(RR-7):1-45.
- CDC. Use of hepatitis B vaccination for adults with diabetes mellitus. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. 50):1709-11.
- Institute of Medicine. 2012. Adverse effects of vaccines: Evidence and causality. Washington D.C. The National Academies Press.
- Institute of Medicine. 2002. Immunization Safety Review: Hepatitis B Vaccine and Demyelinating Neurological Disorders. Washington D.C. The National Academy Press.
- Lewis E, Shinefield HR, Woodruff BA, et al. Safety of neonatal hepatitis B vaccine administration. Pediatr Infect Dis J 2001;20:1049–54.
- Van Damme P, Ward J, Shouval D, Wiersma S, Zanetti A. Hepatitis B vaccine. In: Plotkin SA, Orenstein WA, Offit P, eds. Vaccines. 6th edition. China: Saunders; 2013.
- Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med 2004;351:2832–8.
- Page last reviewed: November 15, 2016
- Page last updated: March 16, 2017
- Content source:


 ShareCompartir
ShareCompartir