Influenza
Printer friendly version [22 pages]
On this Page
Influenza
- Highly infectious viral illness
- First pandemic in 1580
- At least 4 pandemics in 19th century
- Estimated 21 million deaths worldwide in pandemic of 1918-1919
- Virus first isolated in 1933
Influenza Virus
- Single-stranded RNA virus
- Orthomyxoviridae family
- 3 types: A,B,C
- Subtypes of type A determined by hemagglutinin and neuraminidase
Influenza Virus Strains
- Type A-moderate to severe illness
- all age groups
- humans and other animals
- Type B-milder disease
- primarily affects children
- humans only
- Type C-rarely reported in humans
- no epidemics
Influenza Virus
Influenza is a highly infectious viral illness. The name “influenza” originated in 15th century Italy, from an epidemic attributed to “influence of the stars.” The first pandemic, or worldwide epidemic, that clearly fits the description of influenza was in 1580. At least four pandemics of influenza occurred in the 19th century, and three occurred in the 20th century. The pandemic of “Spanish” influenza in 1918–1919 caused an estimated 21 million deaths worldwide. The first pandemic of the 21st century occurred in 2009–2010.
Smith, Andrewes, and Laidlaw isolated influenza A virus in ferrets in 1933, and Francis isolated influenza B virus in 1936. In 1936, Burnet discovered that influenza virus could be grown in embryonated hens’ eggs. This led to the study of the characteristics of the virus and the development of inactivated vaccines. The protective efficacy of these inactivated vaccines was determined in the 1950s. The first live attenuated influenza vaccine was licensed in 2003.
Influenza Virus
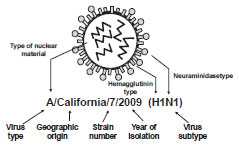
Influenza is a single-stranded, helically shaped, RNA virus of the orthomyxovirus family. Basic antigen types A, B, and C are determined by the nuclear material. Type A influenza has subtypes that are determined by the surface antigens hemagglutinin (H) and neuraminidase (N). Three types of hemagglutinin in humans (H1, H2, and H3) have a role in virus attachment to cells. Two types of neuraminidase (N1 and N2) have a role in virus penetration into cells.
Influenza A causes moderate to severe illness and affects all age groups. The virus infects humans and other animals. Influenza A viruses are perpetuated in nature by wild birds, predominantly waterfowl. Most of these viruses are not pathogenic to their natural hosts and do not change or evolve. Influenza B generally causes milder disease than type A and primarily affects children. Influenza B is more stable than influenza A, with less antigenic drift and consequent immunologic stability. It affects only humans. Influenza C is rarely reported as a cause of human illness, probably because most cases are subclinical. It has not been associated with epidemic disease.
The nomenclature to describe the type of influenza virus is expressed in this order: 1) virus type, 2) geographic origin where it was first isolated, 3) strain number, 4) year of isolation, and 5) virus subtype.
Antigenic Changes
Influenza Antigenic Changes
- Antigenic Drift
- major change, new subtype
- caused by point mutations in gene
- may result in pandemic
- Antigenic Shift
- major change, new subtype
- caused by exchange of gene segments
- may result in pandemic
Hemagglutinin and neuraminidase periodically change, apparently due to sequential evolution within immune or partially immune populations. These changes may take the form of antigenic drift or antigenic shift, the latter associated with pandemics.
In antigenic drift, antigenic mutants emerge and are selected as the predominant virus to the extent that they differ from the antecedent virus, which is suppressed by specific antibody arising in the population as a result of infection. This cycle repeats continuously. In interpandemic periods, mutants arise by serial point mutations in the RNA coding for hemagglutinin.
Antigenic drift is a minor change in surface antigens that results from point mutations in a gene segment. Antigenic drift may result in an epidemic, since the protection that remains from past exposures to similar viruses is incomplete. Drift occurs in all three types of influenza virus (A,B,C). For instance, during most of the 1997–1998 influenza season, A/Wuhan/359/95 (H3N2) was the predominant influenza strain isolated in the United States. A/Wuhan was a drifted distant relative of the 1968 Hong Kong H3N2 strain. In the last half of the 1997–1998 influenza season, a drifted variant of A/Wuhan appeared. This virus, named A/Sydney/5/97, was different enough from A/Wuhan (which had been included in the 1997–1998 vaccine) that the vaccine did not provide much protection. Both A/Wuhan and A/Sydney circulated late in the 1997–1998 influenza season. A/Sydney became the predominant strain during the 1998–1999 influenza season and was included in the 1998–1999 vaccine. In antigenic shift, at irregular intervals of 10 to >40 years, viruses showing major antigenic differences from prevalent subtypes appear and, because the population does not have protective antibody against these new antigens, cause pandemic disease. Antigenic shift involves a major change in one or both surface antigens (H or N). Antigenic shifts are probably due to genetic recombination (an exchange of a gene segment) between influenza A viruses that affect humans and/or animals. An antigenic shift may result in a worldwide pandemic if the virus is efficiently transmitted from person to person. An antigenic shift occurred in 1968 when H3N2 (Hong Kong) influenza appeared. It completely replaced the type A strain (H2N2, or Asian influenza) that had circulated throughout the world for the prior 10 years.
Since the late 19th century, five occurrences of antigenic shifts have led to pandemics (1889–1891, 1918–1920, 1957–1958, 1968–1969, and 2009-2010). A pandemic may start from a single focus and spread along routes of travel. Typically, there are high attack rates involving all age groups, and mortality is usually markedly increased. Severity is generally not greater in the individual patient (except for the 1918–1919 strain), but because large numbers of persons are infected, the number, if not the proportion, of severe and fatal cases will be large. Onset may occur in any season of the year. Secondary and tertiary waves may occur up to 2 years later, usually in the winter.
2009 Influenza A(H1N1)
- In April 2009 a novel influenza A(H1N1) virus appeared and quickly spread across North America
- By May 2009 the virus had spread to many areas of the world
- Cause of the first influenza pandemic since 1968
- Pandemic monovalent influenza vaccine produced and deployed in nationwide vaccination campaign
In April 2009, a novel influenza A(H1N1) virus appeared and quickly spread across North America. By May 2009 the virus had spread to many areas of the world. Influenza morbidity caused by 2009 pandemic H1N1 virus remained above seasonal baselines throughout spring and summer 2009 and was the cause of the first influenza pandemic since 1968.
In the United States, the 2009 pandemic was characterized by a substantial increase in influenza activity in Spring 2009 that was well beyond seasonal norms. Influenza activity peaked in late October 2009, and returned to the seasonal baseline by January 2010. During this time, more than 99 percent of viruses characterized were the 2009 pandemic influenza A(H1N1) virus.
In January 2011, CDC estimated that pandemic H1N1 influenza virus caused more than 60 million Americans to become ill, and led to more than 270,000 hospitalizations and 12,500 deaths. Ninety percent of hospitalizations and deaths occurred in persons younger than 65 years of age. With typical seasonal influenza approximately 90% of deaths occur in persons older than 65 years.
In response to the pandemic a monovalent influenza vaccine was produced and deployed in a nationwide vaccination campaign.
Typically in an epidemic, influenza attack rates are lower than in pandemics. The major impact is observed in morbidity, with high attack rates and excess rates of hospitalization, especially for adults with respiratory disease. Absenteeism from work and school is high, and visits to healthcare providers increase. In the Northern Hemisphere, epidemics usually occur in late fall and continue through early spring. In the Southern Hemisphere, epidemics usually occur 6 months before or after those in the Northern Hemisphere.
Sporadic outbreaks can occasionally be localized to families, schools, and isolated communities.
Pathogenesis
Influenza Pathogenesis
- Respiratory transmission of virus
- Replication in respiratory epithelium with subsequent destruction of cells
- Viremia rarely documented
- Virus shed in respiratory secretions for 5-10 days
Influenza Clinical Features
- Incubation period 2 days (range 1-4 days)
- 50% of infected persons develop classic symptoms
- Abrupt onset of fever, myalgia, sore throat, nonproductive cough, headache
Following respiratory transmission, the virus attaches to and penetrates respiratory epithelial cells in the trachea and bronchi. Viral replication occurs, which results in the destruction of the host cell. Viremia has rarely been documented. Virus is shed in respiratory secretions for 5–10 days.
Clinical Features
The incubation period for influenza is usually 2 days, but can vary from 1 to 4 days. Influenza illness can vary from asymptomatic infection to severe. In general, only about 50% of infected persons will develop the classic clinical symptoms of influenza.
“Classic” influenza disease is characterized by the abrupt onset of fever, myalgia, sore throat, nonproductive cough, and headache. The fever is usually 101°–102°F, and accompanied by prostration (bedridden). The onset of fever is often so abrupt that the exact hour is recalled by the patient. Myalgias mainly affect the back muscles. Cough is believed to be a result of tracheal epithelial destruction. Additional symptoms may include rhinorrhea (runny nose), headache, substernal chest burning and ocular symptoms (e.g., eye pain and sensitivity to light).
Systemic symptoms and fever usually last from 2 to 3 days, rarely more than 5 days. They may be decreased by such medications as aspirin or acetaminophen. Aspirin should not be used for infants, children, or teenagers because they may be at risk for contracting Reye syndrome following an influenza infection. Recovery is usually rapid, but some patients may have lingering asthenia (lack of strength or energy) for several weeks.
Complications
Influenza Complications
- Pneumonia
- secondary bacterial
- primary influenza viral
- Reye syndrome
- Myocarditis
- Death is reported than less than 1 per 1,000 cases
The most frequent complication of influenza is pneumonia, most commonly secondary bacterial pneumonia (e.g., Streptococcus pneumoniae, Haemophilus influenzae, or Staphylococcus aureus). Primary influenza viral pneumonia is an uncommon complication with a high fatality rate. Reye syndrome is a complication that occurs almost exclusively in children taking aspirin, primarily in association with influenza B (or varicella zoster), and presents with severe vomiting and confusion, which may progress to coma due to swelling of the brain.
Other complications include myocarditis (inflammation of the heart) and worsening of chronic bronchitis and other chronic pulmonary diseases. Death is reported in less than 1 per 1,000 cases. The majority of deaths typically occur among persons 65 years of age and older.
Impact of Influenza
Impact of Influenza - United States, 1976-2007
- The number of influenza-associated deaths varies substantially by year, influenza virus type and subtype, and age group
- Annual influenza-associated deaths ranged from 3,349 (1985-86 season) to 48,614 (2003-04 season), with an average of 23,607 annual deaths
- Persons 65 years of age and older account for approximately 90% of deaths
- 2.7 times more deaths occurred during seasons when A(H3N2) viruses were prominent
An increase in mortality typically accompanies an influenza epidemic. Increased mortality results not only from influenza and pneumonia but also from cardiopulmonary and other chronic diseases that can be exacerbated by influenza.
The number of influenza-associated deaths varies substantially by year, influenza virus type and subtype, and age group. In a study of influenza seasons from 1976-77 through 2006-07, the estimated number of annual influenza-associated deaths from respiratory and circulatory causes ranged from a low of 3,349 (1985-86 season) to a high of 48,614 (2003-04 season), with an average of 23,607 annual influenza-associated deaths. Persons 65 years of age and older account for approximately 90% of deaths attributed to pneumonia and influenza. During seasons with prominent circulation of influenza A(H3N2) viruses, 2.7 times more deaths occurred than during seasons when A(H3N2) viruses were not prominent.
The risk for complications and hospitalizations from influenza are higher among persons 65 years of age and older, young children, and persons of any age with certain underlying medical conditions. An average of more than 200,000 hospitalizations per year are related to influenza, with about 37% occurring in persons younger than 65 years. A greater number of hospitalizations occur during years that influenza A(H3N2) is predominant. In nursing homes, attack rates may be as high as 60%, with fatality rates as high as 30%. The cost of a severe epidemic has been estimated to be $12 billion.
Impact of Influenza - United States
- Highest rates of complications and hospitalization among persons 65 years and older, young children, and persons of any age with certain underlying medical conditions
- Average of more than 200,000 influenza-related excess hospitalizations
- 37% of hospitalizations among persons younger than 65 years of age
- Greater number of hospitalizations during years that A(H3N2) is predominant
Among children 0–4 years of age, hospitalization rates have varied from 100 per 100,000 healthy children to as high as 500 per 100,000 for children with underlying medical conditions. Hospitalization rates for children 24 months of age and younger are comparable to rates for persons 65 and older. Children 24-59 months of age are at less risk of hospitalization from influenza than are younger children, but are at increased risk for influenza-associated clinic and emergency department visits.
Healthy children 5 through 18 years of age are not at increased risk of complications of influenza. However, children typically have the highest attack rates during community outbreaks of influenza. They also serve as a major source of transmission of influenza within communities. Influenza has a substantial impact among school-aged children and their contacts. These impacts include school absenteeism, medical care visits, and parental work loss. Studies have documented 5 to 7 influenza-related outpatient visits per 100 children annually, and these children frequently receive antibiotics.
Top of PageLaboratory Diagnosis
Influenza Among School-Aged Children
- School-aged children
- typically have the highest attack rates during community outbreaks of influenza
- serve as a major source of transmission of influenza within communities
Influenza Diagnosis
- Clinical and epidemiological characteristics
- Isolation of influenza virus from clinical specimen (e.g., throat, nasopharynx, sputum)
- Significant rise in influenza IgG by serologic assay
The diagnosis of influenza is usually suspected on the basis of characteristic clinical findings, particularly if influenza has been reported in the community.
Virus can be isolated from throat and nasopharyngeal swabs obtained within 3 days of onset of illness. Culture is performed by inoculation of the amniotic or allantoic sac of chick embryos or certain cell cultures that support viral replication. A minimum of 48 hours is required to demonstrate virus, and 1 to 2 additional days to identify the virus type. As a result, culture is helpful in defining the etiology of local epidemics, but not in individual case management.
Serologic confirmation of influenza requires demonstration of a significant rise in influenza IgG. The acute-phase specimen should be taken less than 5 days from onset, and a convalescent specimen taken 10–21 days (preferably 21 days) following onset. Complement fixation (CF) and hemagglutination inhibition (HI) are the serologic tests most commonly used. The key test is HI, which depends on the ability of the virus to agglutinate erythrocytes and inhibition of this process by specific antibody. Diagnosis requires at least a fourfold rise in antibody titer. Rapid diagnostic testing for influenza antigen is available, but because these tests fail to detect many patients with influenza, CDC recommends antiviral treatment with oseltamivir or zanamivir as early as possible for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at greater risk for serious influenza-related complications.
Details about the laboratory diagnosis of influenza are available on the CDC influenza website.
Epidemiology
Influenza Epidemiology
- Reservoir
- Human, animals (type A only)
- Transmission
- respiratory
- probably airborne
- Temporal pattern
- peak December - March in temperate climate
- may occur earlier or later
- Communicability
- 1 day before to 5 days after onset (adults)
Occurrence
Influenza occurs throughout the world.
Reservoir
Humans are the only known reservoir of influenza types B and C. Influenza A viruses may infect both humans and animals. There is no chronic carrier state.
Transmission
Influenza is primarily transmitted from person to person via large virus-laden droplets (particles more than 5 microns in diameter) that are generated when infected persons cough or sneeze. These large droplets can then settle on the mucosal surfaces of the upper respiratory tracts of susceptible persons who are near (within 3 feet) infected persons. Transmission may also occur through direct contact or indirect contact with respiratory secretions such as when touching surfaces contaminated with influenza virus and then touching the eyes, nose or mouth.
Temporal Pattern
Influenza activity peaks from December to March in temperate climates, but may occur earlier or later. During 1982–2012, peak influenza activity in the United States occurred most frequently in January (17% of seasons), and February (47% of seasons). However, peak influenza activity occurred in March, April, or May in 17% of seasons. Influenza occurs throughout the year in tropical areas.
Communicability
Adults can transmit influenza from the day before symptom onset to approximately 5 days after symptoms begin. Children can transmit influenza to others for 10 or more days.
Secular Trends in the United States
Pneumonia and Influenza Mortality for 122 U.S. Cities
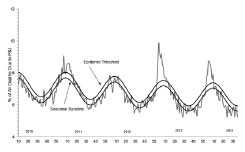
There is a documented association between influenza and increased morbidity in high-risk adult populations. Hospitalization for adults with high-risk medical conditions increases two- to fivefold during major epidemics.
The impact of influenza in the United States is quantified by measuring pneumonia and influenza (P and I) deaths. Death certificate data are collected from 122 U.S. cities with populations of more than 100,000 (total of approximately 70,000,000). P and I deaths include all deaths for which pneumonia is listed as a primary or underlying cause or for which influenza is listed on the death certificate.
An expected ratio of deaths due to P and I compared with all deaths for a given period of time is determined. The epidemic threshold for influenza seasons is generally estimated at 1.645 standard deviations above observed P and I deaths for the previous 5-year period excluding periods during influenza outbreaks. Influenza epidemic activity is signaled when the ratio of deaths due to P and I exceeds the threshold ratio for 2 consecutive weeks.
Influenza Vaccine
Characteristics
Influenza Vaccines
- Inactivated subunit (IIV)
- intramuscular or intradermal
- Live attenuated vaccine (LAIV)
- intranasal
Two types of influenza vaccine are available in the United States, inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV). IIV has been available since the 1940s. IIV is administered by the intramuscular or intradermal route. Trivalent vaccine contains three inactivated viruses: type A(H1N1), type A(H3N2), and type B. Quadrivalent influenza vaccines were introduced for the 2013-2014 season. They contain the same antigens as trivalent vaccines, with the exception that quadrivalent vaccines contain two type B strains. Only split-virus and subunit inactivated vaccines are available in the United States. Vaccine viruses are grown in chicken eggs, and the final product contains residual egg protein. The vaccine is available in both pediatric (0.25-mL dose) and adult (0.5-mL dose) formulations.
Multiple manufacturers produce inactivated influenza vaccine each year for the U.S. market. Vaccines are available in multiple presentations (single dose syringes and vials, multi-dose vials) and in preservative-free formulations. Approved age indications vary by manufacturer and product. Clinicians should obtain inactivated influenza vaccine appropriate for the age groups they plan to vaccinate. ACIP does not recommend use of influenza vaccine outside the vaccine’s FDA-approved age indication. Tables listing each year’s influenza vaccines are available in the annual ACIP influenza statement, and on the CDC influenza website.
Transmission of LAIV Virus
- LAIV replicates in the nasopharyngeal mucosa
- Vaccinated children can shed vaccine viruses in nasopharyngeal secretions for up to 3 weeks
- One instance of transmission of vaccine virus to a contact has been documented
Inactivated Influenza Vaccine Efficacy
- About 60% effective among healthy persons younger than 65 years of age
- 50-60% effective in preventing hospitalization among elderly persons
- 80% effective in preventing death among elderly persons
Influenza and Complications Among Nursing Home Residents
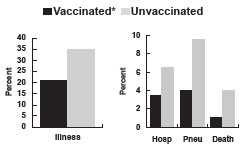
*Inactivated influenza vaccine. Genesee County, MI, 1982-1983
LAIV Efficacy in Healthy Children
- 87% effective against culture-confirmed influenza in children 60 – 84 months old
- 27% reduction in febrile otitis media (OM)
- 28% reduction in OM with accompanying antibiotic use
- Decreased fever and OM in vaccine recipients who developed influenza
Flucelvax was approved by the FDA in November 2012. It is a trivalent subunit IIV prepared from virus propagated in Madin Darby Canine Kidney (MDCK) cells. It is a cell-culture inactivated influenza vaccine (ccIIV). It is approved for persons 18 years old or older.
One inactivated influenza vaccine product, FluBlok, is a recombinant influenza vaccine (RIV). It is trivalent, administered by intramuscular injection, and is indicated for persons aged 18 through 49 years. RIV is manufactured without the use of influenza viruses; therefore, similarly to IIVs, no shedding of vaccine virus will occur. No preference is expressed for RIV vs. IIV within specified indications.
In 2009 the Food and Drug Administration (FDA) approved a new formulation of inactivated influenza vaccine produced by sanofi pasteur, brand name Fluzone High-Dose. This vaccine is approved only for persons 65 years of age or older. Each dose of this vaccine contains 4 times as much hemagglutinin as the regular formulation of Fluzone for adults. ACIP has not expressed a preference for the high dose Fluzone formulation or any other inactivated vaccine for use in persons 65 years and older.
In 2011 the FDA approved a IIV formulation administered by the intradermal route. The product is Fluzone Intradermal produced by sanofi pasteur. It is approved for persons 18 through 64 years of age. This vaccine formulation is not the same as intramuscular IIV preparations. Each 0.1 mL dose contains 27 micrograms of hemagglutinin. The vaccine is administered with a specially designed prefilled syringe with a 30 gauge 1.5 millimeter microneedle.
In 2014, the FDA approved Alfuria influenza vaccine to be administered by the Stratis® Jet Injector. FDA approved this method of administration for adults 18 through 64 years of age.
Live attenuated influenza vaccine (LAIV) was approved for use in the United States in 2003. It contains the same influenza viruses as IIV. The viruses are cold-adapted, and replicate effectively in the mucosa of the nasopharynx. The vaccine viruses are grown in chicken eggs, and the final product contains residual egg protein. The vaccine is provided in a single-dose sprayer unit; half of the dose is sprayed into each nostril. LAIV does not contain thimerosal or any other preservative. LAIV is approved for use only in healthy, nonpregnant persons 2 through 49 years of age.
Vaccinated children can shed vaccine viruses in nasopharyngeal secretions for up to 3 weeks. One instance of transmission of vaccine virus to a contact has been documented.
Immunogenicity and Vaccine Efficacy
IIV
For practical purposes, the duration of immunity following inactivated influenza vaccination is less than 1 year because of waning of vaccine-induced antibody and antigenic drift of circulating influenza viruses. Influenza vaccine efficacy varies by the similarity of the vaccine strain(s) to the circulating strain and the age and health status of the recipient. Vaccines are effective in protecting about 60% of healthy vaccinees younger than 65 years of age from illness when the vaccine strain is similar to the circulating strain. However, the vaccine is less effective in preventing illness among persons 65 years of age and older.
Although the vaccine is not highly effective in preventing clinical illness among the elderly, it is effective in preventing complications and death. Some studies show that, among elderly persons, the vaccine is 50%–60% effective in preventing hospitalization and 80% effective in preventing death. During a 1982–1983 influenza outbreak in Genesee County, Michigan, unvaccinated nursing home residents were four times more likely to die than were vaccinated residents.
LAIV
Inactivated Influenza Vaccine Recommendations
- Advisory Committee on Immunization Practices recommends annual influenza vaccination for all persons 6 months of age and older
- Protection of persons at higher risk for influenza-related complications should continue to be a focus of vaccination efforts as providers and programs transition to routine vaccination of all persons aged 6 months and older
Inactivated Influenza Vaccine Recommendations
- When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following groups of persons:
- children 6 months through 4 years (59 months) of age
- persons 50 years and older
- persons with chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus)
- persons who are immunosuppressed (including immunosuppression caused by medications or by human immunodeficiency virus)
- women who are or will be pregnant during the influenza season
- children 6 months through 18 years of age and receiving long-term aspirin therapy and who therefore might be at risk for experiencing Reye syndrome after influenza virus infection
- residents of nursing homes and other chronic-care facilities
- American Indians/Alaska Natives
- persons who are morbidly obese (body-mass index is 40 or greater)
- healthcare personnel
- household contacts and caregivers of children younger than 5 years of age and adults 50 years of age or older, with particular emphasis on vaccinating contacts of children aged younger than 6 months
- household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza
Pregnancy and Inactivated Influenza Vaccine
- Risk of hospitalization 4 times higher than nonpregnant women
- Risk of complications comparable to nonpregnant women with high-risk medical conditions
- Vaccination (with IIV) recommended if pregnant during influenza season
- Vaccination can occur during any trimester
LAIV has been tested in groups of both healthy children and healthy adults. A randomized, double-blind, placebo-controlled trial among healthy children 60–84 months of age assessed the efficacy of the trivalent LAIV against culture-confirmed influenza during two influenza seasons. In year 1, when vaccine and circulating virus strains were well matched, efficacy was 87% against culture-confirmed influenza. In year 2, when the type A component was not well matched between vaccine and circulating virus strains, efficacy was also 87%. Other results from this trial included a 27% reduction in febrile otitis media and a 28% reduction in otitis media with concomitant antibiotic use. Receipt of LAIV also resulted in decreased fever and otitis media in vaccine recipients who developed influenza.
A randomized, double-blind, placebo-controlled trial among 3,920 healthy working adults aged 18–49 years assessed several endpoints and documented reductions in illness, absenteeism, healthcare visits, and medication use during influenza outbreak periods. This study was conducted during the 1997–98 influenza season, when the vaccine and circulating type A strains were not well matched. This study did not include laboratory virus testing of cases. Some studies among children have demonstrated greater efficacy for LAIV compared to IIV. There is no evidence in adults that efficacy of LAIV is greater than that of IIV.
Vaccination Schedule and Use
IIV
Influenza activity peaks in temperate areas between late December and early March. IIV should be offered as soon as it becomes available.
Organized vaccination campaigns generally should be scheduled no earlier than mid-October. Although most influenza vaccination activities should be completed by December (particularly for high-risk groups), providers should continue to provide vaccine throughout influenza season.
One dose of IIV may be administered annually for persons 9 years of age or older. Children 6 months through 8 years of age receiving influenza vaccine for the first time should receive two doses administered at least 28 days apart.
In addition, certain children 6 months through 8 years of age who previously received influenza vaccine may be recommended to receive a second dose. Refer to the current ACIP influenza recommendations for guidance on this issue.
Inactivated influenza vaccine should be given by the intramuscular (IM) or intradermal route (Fluzone Intradermal only). Other methods, such as subcutaneous, topical, or mucosal should not be used unless approved by the Food and Drug Administration or recommended by ACIP.
Beginning in the 2010-2011 influenza season the Advisory Committee on Immunization Practices recommended annual influenza vaccination for all persons 6 months of age and older. Protection of persons at higher risk for influenza-related complications should continue to be a focus of vaccination efforts as providers and programs transition to routine vaccination of all persons aged 6 months and older.
When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following groups of persons: children 6 months–4 years (59 months) of age; persons 50 years and older; persons with chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus); persons who are immunosuppressed (including immunosuppression caused by medications or by human immunodeficiency virus); women who are or will be pregnant during the influenza season; children 6 months through 18 years of age and receiving long-term aspirin therapy and who therefore might be at risk for experiencing Reye syndrome after influenza virus infection; residents of nursing homes and other chronic-care facilities; American Indians/Alaska Natives; persons who are morbidly obese (body-mass index is 40 or greater); healthcare personnel; household contacts and caregivers of children younger than 5 years of age and adults 50 years of age and older, with particular emphasis on vaccinating contacts of children aged younger than 6 months; and household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza.
Case reports and limited studies suggest that pregnant women may be at increased risk for serious medical complications of influenza as a result of increases in heart rate, stroke volume and oxygen consumption; decreases in lung capacity; and changes in immunologic function. A study found that the risk of hospitalization for influenza-related complications was more than four times higher for women in the second or third trimester of pregnancy than for nonpregnant women. The risk of complications for these pregnant women was comparable to that for nonpregnant women with high-risk medical conditions, for whom influenza vaccine has been traditionally recommended.
ACIP recommends vaccination of women who will be pregnant during influenza season. Vaccination can occur during any trimester. Influenza season in the United States generally occurs in December through March. Only IIV should be administered to pregnant women.
Available data suggest that persons with HIV infection may have prolonged influenza illnesses and are at increased risk of complications of influenza. Many persons with HIV infection will develop protective antibody titers following inactivated influenza vaccine. In persons who have advanced HIV disease and low CD4+ T-lymphocyte cell counts, IIV vaccine may not induce protective antibody titers. A second dose of vaccine does not improve the immune response in these persons.
HIV Infection and Inactivated Influenza Vaccine
- Persons with HIV at increased risk of complications of influenza
- IIV induces protective antibody titers in many HIV-infected persons
- TIV will benefit many HIV-infected persons
Efforts should be made to vaccinate household and other close contacts of high-risk persons. These include healthcare personnel, employees of long-term care facilities, and household contacts of high-risk persons. These individuals may be younger and healthier and more likely to be protected from illness than are elderly persons. All healthcare providers should receive annual inactivated influenza vaccine. Groups that should be targeted include physicians, nurses, and other personnel in hospitals and outpatient settings who have contact with high-risk patients in all age groups, and providers of home care to high-risk persons (e.g., visiting nurses, volunteers). LAIV may be administered to healthy healthcare personnel 49 years of age or younger, except those who have contact with severely immunosuppressed persons who require hospitalization and care in a protective environment (i.e., in isolation because of severe immunosuppression).
LAIV
Simultaneous Administration of LAIV and Other Vaccines
- Inactivated vaccines can be administered either simultaneously or at any time before or after LAIV
- Other live vaccines can be administered on the same day as LAIV
- Live vaccines not administered on the same day should be administered at least 4 weeks apart
LAIV is approved for healthy, nonpregnant persons 2 through 49 years of age. The vaccine can be administered to eligible persons as soon as it becomes available in the late summer or fall. Vaccination can continue throughout influenza season. One dose of LAIV may be administered by the intranasal route to persons 9 through 49 years of age. Children 2 through 8 years of age receiving influenza vaccine for the first time should receive two doses administered at least 4 weeks apart.
In addition, certain children 6 months through 8 years of age who previously received influenza vaccine may be recommended to receive a second dose. Refer to the current ACIP influenza recommendations for guidance on this issue.
Close contacts of persons at high risk for complications from influenza should receive influenza vaccine. Contacts of persons at high risk of complications of influenza may receive LAIV if they are otherwise eligible (i.e., 2 through 49 years of age, healthy and not pregnant). Persons in close contact with severely immunosuppressed persons who are hospitalized and receiving care in a protected environment should not receive LAIV.
Inactivated vaccines do not interfere with the immune response to live vaccines. Inactivated vaccines, such as tetanus and diphtheria toxoids, can be administered either simultaneously or at any time before or after LAIV. Other live vaccines can be administered on the same day as LAIV. Live vaccines not administered on the same day should be administered at least 4 weeks apart.
Contraindications and Precautions to Vaccination
Inactivated Influenza Vaccine Contraindications and Precautions
- Severe allergic reaction (e.g., anaphylaxis) to a vaccine component or following a prior dose of inactivated influenza
- Moderate or severe acute illness
- History of Guillian-Barré syndrome (GBS) within 6 weeks following a previous dose of influenza vaccine
Live Attenuated Influenza Vaccine Contraindications, Precautions or Conditions in Persons Who Should Not Receive Influenza Vaccine
- Children younger than 2 years of age, or 50 years of age and older*
- Persons with chronic medical conditions*
- Children and adolescents receiving long-term aspirin or aspirin-containing therapy*
- Immunosuppression from any cause*
- Pregnant women*
- History of egg allergy*
- History of severe allergic reaction following dose of influenza vaccine
- Severe allergy to vaccine component
- History of Guillain-Barré syndrome (GBS) within 6 weeks following a previous dose of influenza vaccine
- Children younger than 5 years with recurrent wheezing*
- Recent wheezing*
- Persons with asthma*
- Persons who care for severely immunosuppressed persons requiring protective environment for 7 days after receipt*
- Persons who have taken influenza antiviral medications within previous 48 hours*
- Moderate or severe acute illness
*These persons should receive inactivated influenza vaccine
IIV
Persons with a severe allergic reaction (anaphylaxis) to a vaccine component or following a prior dose of inactivated influenza vaccine should not receive IIV. In 2011, ACIP revised its recommendation for influenza vaccination of persons with egg allergy. Persons whose allergy involves only urticaria without other symptoms may receive IIV. See the ACIP influenza vaccine recommendations for further information. Persons with a moderate or severe acute illness normally should not be vaccinated until their symptoms have decreased. A history of Guillain-Barré syndrome (GBS) within 6 weeks following a previous dose of influenza vaccine is a precaution for IIV. Pregnancy, breastfeeding, and immunosuppression are not contraindications to inactivated influenza vaccination.
LAIV
Persons who should not receive LAIV include children younger than 2 years of age; persons 50 years of age and older; persons with chronic medical conditions, including asthma, a recent wheezing episode, reactive airways disease or other chronic pulmonary or cardiovascular conditions, metabolic disease such as diabetes, renal disease, or hemoglobinopathy, such as sickle cell disease; and children or adolescents receiving long-term therapy with aspirin or aspirin-containing therapy, because of the association of Reye syndrome with wild-type influenza infection. Persons in these groups should receive inactivated influenza vaccine.
As with other live-virus vaccines, LAIV should not be given to persons who are immunosuppressed because of disease, including HIV, or who are receiving immunosuppressive therapy. Pregnant women should not receive LAIV. Immunosuppressed persons and pregnant women should receive inactivated influenza vaccine. Since LAIV contains residual egg protein, it should not be administered to persons with a history of severe allergy to egg or any other vaccine component. A history of Guillain-Barré syndrome (GBS) within 6 weeks following a previous dose of influenza vaccine is a precaution for LAIV.
As with all vaccines, LAIV should be deferred for persons with a moderate or severe acute illness. If clinical judgment indicates that nasal congestion might impede delivery of the vaccine to the nasopharyngeal mucosa, deferral of administration should be considered until the condition has improved.
The effect on safety and efficacy of LAIV coadministration with influenza antiviral medications has not been studied. However, because influenza antiviral agents reduce replication of influenza viruses, LAIV should not be administered until 48 hours after cessation of influenza antiviral therapy, and influenza antiviral medications should not be administered for 2 weeks after receipt of LAIV.
Adverse Events Following Vaccination
IIV
Local reactions are the most common adverse events following vaccination with IIV.
Although the incidence of Guillain-Barré syndrome (GBS) in the general population is very low, persons with a history of GBS have a substantially greater likelihood of subsequently developing GBS than do persons without such a history, irrespective of vaccination. As a result, the likelihood of coincidentally developing GBS after influenza vaccination is expected to be greater among persons with a history of GBS than among persons with no history of GBS. Whether influenza vaccination might be causally associated with this risk for recurrence is not known. It seems prudent for persons known to have developed GBS within 6 weeks of a previous influenza vaccination to avoid subsequent influenza vaccination. For most persons with a history of GBS who are at high risk for severe complications from influenza, the established benefits of influenza vaccination justify yearly vaccination. Unlike the 1976 swine influenza vaccine, subsequent inactivated vaccines prepared from other virus strains have not been clearly associated with an increased frequency of Guillain-Barré syndrome (GBS). However, obtaining a precise estimate of a small increase in risk is difficult for a rare condition such as GBS, which has an annual background incidence of only one to two cases per year per 100,000 adult population.
Top of PageInfluenza Vaccine Adverse Events
- IIV
- local reactions – common
- Guillain-Barré syndrome – expected to be greater among persons with a history of GBS than among persons with no history of GBS
- LAIV
- nonspecific systemic symptoms – common
LAIV
Among children the most common adverse events are nonspecific systemic symptoms (e.g. runny nose and headaches). However, there have been no significant differences between LAIV and placebo recipients in the proportion with these symptoms. Guillain-Barré syndrome has not been associated with LAIV in post-licensure safety monitoring.
Adverse Reactions Following Vaccination
IIV
Local reactions include soreness, erythema, and induration at the site of injection. These reactions are transient, generally lasting 1 to 2 days. Local reactions are reported in 15%–20% of vaccinees.
Nonspecific systemic symptoms, including fever, chills, malaise, and myalgia, are reported in fewer than 1% of IIV recipients. These symptoms usually occur in those with no previous exposure to the viral antigens in the vaccine. They usually occur within 6–12 hours of IIV vaccination and last 1–2 days. Recent reports indicate that these systemic symptoms are no more common than in persons given a placebo injection.
Inactivated Influenza Vaccine Adverse Reactions
- Local reactions (soreness, redness)
- 15%-20%
- Fever, malaise, myalgia
- less than 1%
- Allergic reactions (hives, angioedema, anaphylaxis)
- rare
Rarely, immediate hypersensitivity, presumably allergic, reactions (such as hives, angioedema, allergic asthma, or systemic anaphylaxis) occur after vaccination with IIV. These reactions probably result from hypersensitivity to a vaccine component. Severe allergic and anaphylactic reactions can occur in response to a number of influenza vaccine components, but such reactions are rare. Most currently available influenza vaccines are prepared by means of inoculation of virus into chicken eggs.
ACIP recommends that persons with egg allergy who report only hives after egg exposure should receive IIV, with several additional safety measures, as summarized below:
- Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive influenza vaccine, with the following additional safety measures
- Because studies published to date involved use of IIV, IIV rather than LAIV should be used;
- Vaccine should be administered by a healthcare provider who is familiar with the potential manifestations of egg allergy; and
- Vaccine recipients should be observed for at least 30 minutes for signs of a reaction after administration of each vaccine dose.
- Persons who report having had reactions to egg involving such symptoms as angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, particularly those that occurred immediately or within a short time (minutes to hours) after egg exposure, are more likely to have a serious systemic or anaphylactic reaction upon reexposure to egg proteins. Before receipt of vaccine, such persons should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.
The potential exists for hypersensitivity reactions to any vaccine component. Although exposure to vaccines containing thimerosal can lead to induction of hypersensitivity, most patients do not develop reactions to thimerosal administered as a component of vaccines, even when patch or intradermal tests for thimerosal indicate hypersensitivity. When it has been reported, hypersensitivity to thimerosal has usually consisted of local delayed-type hypersensitivity reactions.
In 1976 there was a small increased risk of GBS following vaccination with an influenza vaccine made to protect against a swine flu virus. The increased risk was approximately 1 additional case of GBS per 100,000 people who received swine flu vaccine. The Institute of Medicine (IOM) conducted a thorough scientific review of this issue in 2003 and concluded that people who received the 1976 swine influenza vaccine had an increased risk for developing GBS. The exact reason for this association is unknown.
Several studies assessing the risk of GBS after seasonal flu vaccines in the years following the 1976 swine influenza vaccination campaign either have not been associated with an increased risk of GBS or have been associated with a small increase in risk of 1 to 2 cases per million people vaccinated. Studies assessing GBS following the 2009 (H1N1) swine-origin flu vaccine also showed that there is a small increased risk of GBS of about 1-3 cases per million people vaccinated. It is important to keep in mind that severe illness and death can result from influenza, and vaccination is the best way to prevent influenza disease and its complications.
LAIV
Live Attenuated Influenza Vaccine Adverse Reactions
- Children
- no significant increase in URI symptoms, fever, or other systemic symptoms
- increased risk of wheezing in children 6-23 months of age
- Adults
- significantly increased rate of cough, runny nose, nasal congestion, sore throat, and chills reported among vaccine recipients
- no increase in the occurrence of fever
- No serious adverse reactions identified
In a clinical trial, children 6 through 23 months of age had an increased risk of wheezing. An increased risk of wheezing was not reported in older children.
In other clinical trials, among healthy adults, a significantly increased rate of cough, runny nose, nasal congestion, sore throat, and chills was reported among vaccine recipients. These symptoms were reported in 10%–40% of vaccine recipients, a rate 3%–10% higher than reported for placebo recipients. There was no increase in the occurrence of fever among vaccine recipients. No serious adverse reactions have been identified in LAIV recipients, either children or adults.
Few data are available concerning the safety of LAIV among persons at high risk for development of complications of influenza, such as immunosuppressed persons or those with chronic pulmonary or cardiac disease. Therefore, persons at high risk of complications of influenza should not receive LAIV. These persons should continue to receive inactivated influenza vaccine.
Vaccine Storage and Handling
Inactivated influenza vaccines should be maintained at refrigerator temperature between 35°F and 46°F (2°C and 8°C). Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
LAIV is intended for intranasal administration only and should never be administered by injection. LAIV is supplied in a prefilled single-use sprayer containing 0.2 mL of vaccine. Approximately 0.1 mL (i.e., half of the total sprayer contents) is sprayed into the first nostril while the recipient is in the upright position. An attached dose-divider clip is removed from the sprayer to administer the second half of the dose into the other nostril. If the vaccine recipient sneezes after administration, the dose should not be repeated.
Strategies for Improving Influenza Vaccine Coverage
On average, fewer than 50% of persons in high-risk groups receive influenza vaccine each year. By November 2012 only 47.3 percent of pregnant women had received influenza vaccine for the 2012-2013 season. This points to the need for more effective strategies for delivering vaccine to high-risk persons, their healthcare providers, and household contacts. Persons for whom the vaccine is recommended can be identified and immunized in a variety of settings.
In physicians’ offices and outpatient clinics, persons who should receive inactivated influenza vaccine should be identified and their charts marked. IIV use should be promoted, encouraged and recommended beginning in October and continuing through the influenza season. Those without regularly scheduled visits should receive reminders.
In nursing homes and other residential long-term care facilities, immunization with IIV should be routinely provided to all residents at one period of time immediately preceding the influenza season; consent should be obtained at the time of admission.
In acute care hospitals and continuing care centers, persons for whom vaccine is recommended who are hospitalized from October through March should be vaccinated prior to discharge. In outpatient facilities providing continuing care to high-risk patients (e.g., hemodialysis centers, hospital specialty-care clinics, outpatient rehabilitation programs), all patients should be offered IIV shortly before the onset of the influenza season.
Visiting nurses and others providing home care to high-risk persons should identify high-risk patients and administer IIV in the home, if necessary.
In facilities providing services to persons 50 years of age and older (e.g., retirement communities, recreation centers), inactivated influenza vaccine should be offered to all unvaccinated residents or attendees on site. Education and publicity programs should also be conducted in conjunction with other interventions.
For travelers, indications for influenza vaccine should be reviewed prior to travel and vaccine offered, if appropriate.
Administrators of all of the above facilities and organizations should arrange for influenza vaccine to be offered to all personnel before the influenza season. Additionally, household members of high-risk persons and others with whom they will be in contact should receive written information about why they should receive the vaccine and where to obtain it.
Antiviral Agents for Influenza
Influenza Antiviral Agents*
- Amantadine and rimantadine
- Not recommended because of documented resistance in U.S. influenza isolates
- Zanamivir and oseltamivir
- neuraminidase inhibitors
- effective against influenza A and B
- oseltamavir and zanamavir approved for prophylaxis
*see influenza ACIP statement or CDC influenza website for details
In the United States, four antiviral agents are approved for preventing or treating influenza: amantadine, rimantadine, zanamivir, and oseltamivir.
Testing of influenza A isolates from the United States and Canada has demonstrated that many of these viruses are resistant to amantadine and rimantadine. The ACIP recommends that neither amantadine nor rimantadine be used for the treatment or chemoprophylaxis of influenza A in the United States until susceptibility to these antiviral drugs has been re-established.
Zanamivir and oseltamivir are members of a new class of drugs called neuraminidase inhibitors and are active against both influenza type A and type B. Zanamivir is provided as a dry powder that is administered by inhalation. It is approved for treatment of uncomplicated acute influenza A or B in persons 7 years of age and older who have been symptomatic for no more than 48 hours. Oseltamivir is provided as an oral capsule. It is approved for the treatment of uncomplicated influenza A or B in persons 1 year of age and older who have been symptomatic for no more than 48 hours. Zanamivir is approved for prophylaxis of influenza in persons 5 years and older. Oseltamivir is approved for prophylaxis of influenza infection among persons 1 year of age and older.
In 2007-08, a significant increase in the prevalence of oseltamivir resistance was reported among influenza A (H1N1) viruses worldwide. During the 2007-08 influenza season, 10.9% of H1N1 viruses tested in the U.S. were resistant to oseltamivir. During 2008 more than 90% of H1N1 viruses were resistant to oseltamivir. For the 2008-09 influenza season CDC recommends that persons who test positive for influenza A should receive only zanamivir if treatment is indicated. Oseltamivir should be used alone only if recent local surveillance data indicate that circulating viruses are likely to be influenza A (H3N2) or influenza B viruses, which have not been found to be resistant to oseltamivir. Additional information about influenza antiviral treatment is available on the CDC influenza website.
Antiviral agents for influenza are an adjunct to vaccine and are not a substitute for vaccine. Vaccination remains the principal means for preventing influenza-related morbidity and mortality. Additional information on the use of influenza antiviral drugs can be found in the current ACIP statement on influenza vaccine and on the CDC influenza website.
Nosocomial Influenza Control
Influenza Surveillance
- Monitor prevalence of circulating strains and detect new strains
- Estimate influenza-related morbidity, mortality and economic loss
- Rapidly detect outbreaks
- Assist disease control through rapid preventive action
Many patients in general hospitals, and especially in referral centers, are likely to be at high risk for complications of influenza. Hospitalized susceptible patients may acquire influenza from other patients, hospital employees, or visitors. The preferred method of control is to administer inactivated influenza vaccine to high-risk patients and medical personnel.
During community influenza A activity, the use of antiviral prophylaxis may be considered for high-risk patients who were not immunized or were immunized too recently to have protective antibody levels. Antiviral agents may also be considered for unimmunized hospital personnel. Other measures include restricting visitors with respiratory illness, cohorting patients with influenza for 5 days following onset of illness, and postponing elective admission of patients with uncomplicated illness.
Influenza Surveillance
Influenza surveillance is intended to monitor the prevalence of circulating strains and detect new strains necessary for vaccine formulation; estimate influenza-related impact on morbidity, mortality, and economic loss; rapidly detect outbreaks; and assist disease control through rapid preventive action (e.g., chemoprophylaxis of unvaccinated high-risk patients).
CDC receives weekly surveillance reports from the states showing the extent of influenza activity. Reports are classified into four categories: no cases, sporadic, regional (cases occurring in counties collectively contributing less than 50% of a state’s population), widespread (cases occurring in counties collectively contributing 50% or more of a state’s population).
See Weekly surveillance reports
Top of PageAcknowledgement
The editors thank Drs. Lisa Grohskopf, Jerome Tokars, Tom Shimabukuro, and Scott Epperson, CDC for their assistance in updating this chapter.
Selected References
- A special issue of Clinical Infectious Diseases (January 2011) focused on the 2009 H1N1 influenza pandemic.
- Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–67.
- CDC. Estimates of deaths associated with seasonal influenza – United States, 1976-2007. MMWR 2010;59(No. 33):1057-62.Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. [Prevention and Control of Seasonal Influenza with Vaccines Recommendations of the Advisory Committee on Immunization Practices — United States, 2013–2014]. MMWR 2013;62(No. RR-7):[1-46].
- CDC. Influenza vaccination of healthcare personnel. Recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP). MMWR 2006; 55(No. RR-2):1-16.
- Fedson DS, Houck P, Bratzler D. Hospital-based influenza and pneumococcal vaccination: Sutton’s law applied to prevention. Infect Control Hosp Epidemiol 2000;21:692–9.
- Glezen WP, Couch RB. Influenza viruses. In: Evans AS, Kaslow RA, eds. Viral Infections of Humans. Epidemiology and Control. 4th edition. New York, NY: Plenum Medical Book Company;1997:473–505.
- Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009;361:1260-7.
- Murphy KR, Strunk RC. Safe administration of influenza vaccine in asthmatic children hypersensitive to egg protein. J Pediatr 1985;106:931–3.
- Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: a 25 year prospective study. J Infect Dis 2002;185:147–52.
- Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995;333:889–93.
- Saxen H, Virtanen M. Randomized, placebo-controlled double blind study on the efficacy of influenza immunization on absenteeism of healthcare workers. Pediatr Infect Dis J 1999;18:779–83.
- Page last reviewed: November 15, 2016
- Page last updated: September 8, 2015
- Content source:


 ShareCompartir
ShareCompartir