Measles
On this Page
Measles
- Highly contagious viral illness
- First described in 7th century
- Near universal infection of childhood in prevaccination era
- Common and often fatal in developing countries
Measles is an acute viral infectious disease. References to measles can be found from as early as the 7th century. The disease was described by the Persian physician Rhazes in the 10th century as “more to be dreaded than smallpox.”
In 1846, Peter Panum described the incubation period of measles and lifelong immunity after recovery from the disease. Enders and Peebles isolated the virus in human and monkey kidney tissue culture in 1954. The first live attenuated vaccine was licensed for use in the United States in 1963 (Edmonston B strain).
Before a vaccine was available, infection with measles virus was nearly universal during childhood, and more than 90% of persons were immune by age 15 years. Measles is still a common and often fatal disease in developing countries. The World Health Organization estimates there were 145,700 deaths globally from measles in 2013.
Measles Virus
Measles Virus
- Paramyxovirus (RNA)
- Hemagglutinin important surface antigen
- One antigenic type
- Rapidly inactivated by heat, sunlight, acidic pH, ether and trypsin
The measles virus is a paramyxovirus, genus Morbillivirus. It is 120–250 nm in diameter, with a core of single-stranded RNA, and is closely related to the rinderpest and canine distemper viruses. Two membrane envelope proteins are important in pathogenesis. They are the F (fusion) protein, which is responsible for fusion of virus and host cell membranes, viral penetration, and hemolysis, and the H (hemagglutinin) protein, which is responsible for adsorption of virus to cells.
There is only one antigenic type of measles virus. Although studies have documented changes in the H glycoprotein, these changes do not appear to be epidemiologically important (i.e., no change in vaccine efficacy has been observed).
Measles virus is rapidly inactivated by heat, sunlight, acidic pH, ether, and trypsin. It has a short survival time (less than 2 hours) in the air or on objects and surfaces.
Pathogenesis
Measles Pathogenesis
- Respiratory transmission of virus
- Replication in nasopharynx and regional lymph nodes
- Primary viremia 2-3 days after exposure
- Secondary viremia 5-7 days after exposure with spread to tissues
Clinical Features
Measles Clinical Features
- Incubation period 10-12 days
- Prodrome 2-4 days
- stepwise increase in fever to 103°F–105°F
- cough, coryza, conjunctivitis
- Koplik spots (rash on mucous membranes)
- Rash
- 2-4 days after prodrome, 14 days after exposure
- persists 5-6 days
- begins on face and upper neck
- maculopapular, becomes confluent
- fades in order of appearance
The incubation period of measles, from exposure to prodrome, averages 10–12 days. From exposure to rash onset averages 14 days (range, 7–21 days).
The prodrome lasts 2–4 days (range 1–7 days). It is characterized by fever, which increases in stepwise fashion, often peaking as high as 103°F –105°F. This is followed by the onset of cough, coryza (runny nose), or conjunctivitis.
Koplik spots, a rash present on mucous membranes, is considered to be pathognomonic for measles. It occurs 1–2 days before the rash to 1–2 days after the rash, and appears as punctate blue-white spots on the bright red background of the buccal mucosa.
The measles rash is a maculopapular eruption that usually lasts 5–6 days. It begins at the hairline, then involves the face and upper neck. During the next 3 days, the rash gradually proceeds downward and outward, reaching the hands and feet. The maculopapular lesions are generally discrete, but may become confluent, particularly on the upper body. Initially, lesions blanch with fingertip pressure. By 3–4 days, most do not blanch with pressure. Fine desquamation occurs over more severely involved areas. The rash fades in the same order that it appears, from head to extremities.
Other symptoms of measles include anorexia; diarrhea, especially in infants; and generalized lymphadenopathy.
Complications
Measles Complications by Age Group
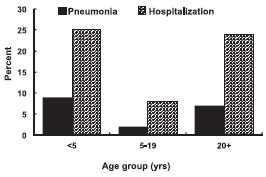
Measles Complications
| Diarrhea | 8% |
|---|---|
| Otitis media | 7% |
| Pneumonia | 6% |
| Encephalitis | 0.1% |
| Seizures | 0.6-0.7% |
| Death | 0.2% |
Based on 1985-1992 surveillance data
Approximately 30% of reported measles cases have one or more complications. Complications of measles are most common among children younger than 5 years of age and adults 20 years of age and older.
From 1985 through 1992, diarrhea was reported in 8% of measles cases, making this the most commonly reported complication of measles. Otitis media was reported in 7% of cases and occurs almost exclusively in children. Pneumonia (in 6% of reported cases) may be viral or superimposed bacterial, and is the most common cause of measles-related death.
Acute encephalitis occurs in approximately 0.1% of reported cases. Onset generally occurs 6 days after rash onset (range 1–15 days) and is characterized by fever, headache, vomiting, stiff neck, meningeal irritation, drowsiness, convulsions, and coma. Cerebrospinal fluid shows pleocytosis and elevated protein. The case-fatality rate is approximately 15%. Some form of residual neurologic damage occurs in as many as 25% of cases. Seizures (with or without fever) are reported in 0.6%–0.7% of cases.
Death from measles was reported in approximately 0.2% of the cases in the United States from 1985 through 1992. As with other complications of measles, the risk of death is highest among young children and adults. Pneumonia accounts for about 60% of deaths. The most common causes of death are pneumonia in children and acute encephalitis in adults.
Subacute sclerosing panencephalitis (SSPE) is a rare degenerative central nervous system disease believed to be due to persistent measles virus infection of the brain. Onset occurs an average of 7 years after measles (range 1 month–27 years), and occurs in five to ten cases per million reported measles cases. The onset is insidious, with progressive deterioration of behavior and intellect, followed by ataxia (awkwardness), myoclonic seizures, and eventually death. SSPE has been extremely rare since the early 1980s.
Measles illness during pregnancy results in a higher risk of premature labor, spontaneous abortion, and low-birthweight infants. Birth defects (with no definable pattern of malformation) have been reported rarely, without confirmation that measles was the cause.
“Atypical measles” occurs only in persons who received inactivated (killed) measles vaccine (KMV) and are subsequently exposed to wild-type measles virus. An estimated 600,000 to 900,000 persons received KMV in the United States from 1963 to 1967. KMV sensitizes the recipient to measles virus antigens without providing protection. Subsequent infection with measles virus leads to signs of hypersensitivity polyserositis. The illness is characterized by fever, pneumonia, pleural effusions, and edema. The rash is usually maculopapular or petechial, but may have urticarial, purpuric, or vesicular components. It appears first on the wrists or ankles. Atypical measles may be prevented by revaccinating with live measles vaccine. Moderate to severe local reactions with or without fever may follow vaccination; these reactions are less severe than with with wild measles virus infection.
Modified measles occurs primarily in patients who received immune globulin (IG) as postexposure prophylaxis and in young infants who have some residual maternal antibody. It is usually characterized by a prolonged incubation period, mild prodrome, and sparse, discrete rash of short duration. Similar mild illness has been reported among previously vaccinated persons.
Rarely reported in the United States, hemorrhagic measles is characterized by high fever (105°F–106°F), seizures, delirium, respiratory distress, and hemorrhage into the skin and mucous membranes.
Measles in an immunocompromised person can be severe with a prolonged course. It is reported almost exclusively in persons with T-cell deficiencies (certain leukemias, lymphomas, and acquired immunodeficiency syndrome [AIDS]). It may occur without the typical rash, and a patient may shed virus for several weeks after the acute illness.
Measles in developing countries has resulted in high attack rates among children younger than 12 months of age. Measles is more severe in malnourished children, particularly those with vitamin A deficiency. Complications include diarrhea, dehydration, stomatitis, inability to feed, and bacterial infections (skin and elsewhere). The case-fatality rate may be as high as 25%. Measles is also a leading cause of blindness in African children.
Laboratory Diagnosis
Measles Laboratory Diagnosis
- Isolation of measles virus from urine, nasopharynx, blood, throat
- Significant rise in measles IgG by any standard serologic assay (e.g., EIA, HI)
- Positive serologic test for measles IgM antibody
Isolation of measles virus is not recommended as a routine method to diagnose measles. However, virus isolates are extremely important for molecular epidemiologic surveillance to help determine the geographic origin of the virus and the viral strains circulating in the United States.
Measles virus can be isolated from urine, nasopharyngeal aspirates, heparinized blood, or throat swabs. Specimens for virus culture should be obtained from every person with a clinically suspected case of measles and should be shipped to the state public health laboratory or CDC, at the direction of the state health department. Clinical specimens for viral isolation should be collected at the same time as samples taken for serologic testing. Because the virus is more likely to be isolated when specimens are collected within 3 days of rash onset, collection of specimens for virus isolation should not be delayed until serologic confirmation is obtained. Clinical specimens should be obtained within 7 days, and not more than 10 days, after rash onset. A detailed protocol for collection of specimens for viral isolation is available on the CDC website.
Serologic testing, most commonly by enzyme-linked immunoassay (EIA), is widely available and may be diagnostic if done at the appropriate time. Generally, a previously susceptible person exposed to either vaccine or wild-type measles virus will first mount an IgM response and then an IgG response. The IgM response will be transient (1–2 months), and the IgG response should persist for many years. Uninfected persons should be IgM negative and will be either IgG negative or IgG positive, depending upon their previous infection or vaccination history.
EIA for IgM antibody requires only a single serum specimen and is diagnostic if positive. The preferred reference test is a capture IgM test developed by CDC. This test should be used to confirm every case of measles that is reported to have some other type of laboratory confirmation. IgM capture tests for measles are often positive on the day of rash onset. However, in the first 72 hours after rash onset, up to 20% of tests for IgM may give false-negative results. Tests that are negative in the first 72 hours after rash onset should be repeated. IgM is detectable for at least 30 days after rash onset and frequently longer.
A variety of tests for IgG antibodies to measles are available and include EIA, hemagglutination inhibition (HI), indirect fluorescent antibody tests, microneutralization, and plaque reduction neutralization. Complement fixation, while widely used in the past, is no longer recommended.
IgG testing for acute measles requires demonstration of a four-fold rise in titer of antibody against measles virus, so two serum specimens are always required. The first specimen should be drawn as soon after rash onset as possible. The second specimen should be drawn 10–30 days later. The tests for IgG antibody should be conducted on both specimens at the same time. The same type of test should be used on both specimens. The specific criteria for documenting an increase in titer depend on the test.
Tests for IgG antibody require two serum specimens, and a confirmed diagnosis cannot be made until the second specimen is obtained. As a result, IgM tests are generally preferred to confirm the diagnosis of measles.
Epidemiology
Measles Epidemiology
- Reservoir
- human
- Transmission
- Respiratory Airborne
- Temporal pattern
- Peak in late winter - spring
- Communicability
- 4 days before to 4 days after rash onset
Occurrence
Measles occurs throughout the world. However, interruption of indigenous transmission of measles has been achieved in the United States and other parts of the Western Hemisphere.
Reservoir
Measles is a human disease. There is no known animal reservoir, and an asymptomatic carrier state has not been documented.
Transmission
Measles transmission is primarily person to person via large respiratory droplets. Airborne transmission via aerosolized droplet nuclei has been documented in closed areas (e.g., office examination room) for up to 2 hours after a person with measles occupied the area.
Temporal Pattern
In temperate areas, measles disease occurs primarily in late winter and spring.
Communicability
Measles is highly communicable, with greater than 90% secondary attack rates among susceptible persons. Measles may be transmitted from 4 days before to 4 days after rash onset. Maximum communicability occurs from onset of prodrome through the first 3–4 days of rash.
Secular Trends in the United States
Measles - United States, 1950-2011
Measles - United States, 1980-2011
Measles Resurgence - United States, 1989-1991
- Cases
- 55,622
- Age group affected
- children younger than five years
- Deaths
- 123
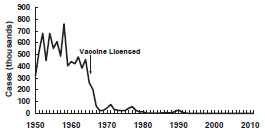
Before 1963, approximately 500,000 cases and 500 deaths were reported annually, with epidemic cycles every 2–3 years. However, the actual number of cases was estimated at 3–4 million annually. More than 50% of persons had measles by age 6, and more than 90% had measles by age 15. The highest incidence was among 5–9-year-olds, who generally accounted for more than 50% of reported cases.
In the years following licensure of vaccine in 1963, the incidence of measles decreased by more than 95%, and 2–3-year epidemic cycles no longer occurred. Because of this success, a 1978 Measles Elimination Program set a goal to eliminate indigenous measles by October 1, 1982 (26,871 cases were reported in 1978). The 1982 elimination goal was not met, but in 1983, only 1,497 cases were reported (0.6 cases per 100,000 population), the lowest annual total ever reported up to that time.
During 1980–1988, a median of 57% of reported cases were among school-aged persons (5–19 years of age), and a median of 29% were among children younger than 5 years of age. A median of 8% of cases were among infants younger than 1 year of age.
From 1985 through 1988, 42% of cases occurred in persons who were vaccinated on or after their first birthday. During these years, 68% of cases in school-aged children (5–19 years) occurred among those who had been appropriately vaccinated. The occurrence of measles among previously vaccinated children (i.e., vaccine failure) led to a recommendation for a second dose in this age group.
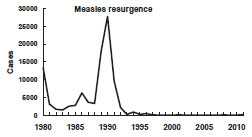
Measles Resurgence in 1989–1991
From 1989 through 1991, a dramatic increase in reported measles cases occurred. During these 3 years a total of 55,622 cases were reported (18,193 in 1989; 27,786 in 1990; 9,643 in 1991). In addition to the increased number of cases, a change occurred in their age distribution. Prior to the resurgence, school-aged children had accounted for the largest proportion of reported cases. During the resurgence, 45% of all reported cases were in children younger than 5 years of age. In 1990, 48% of patients were in this age group, the first time that the proportion of cases in children younger than 5 years of age exceeded the proportion of cases in 5–19-year-olds (35%).
Overall incidence rates were highest for Hispanics and blacks and lowest for non-Hispanic whites. Among children younger than 5 years of age, the incidence of measles among blacks and Hispanics was four to seven times higher than among non-Hispanic whites.
A total of 123 measles-associated deaths were reported during this period (death-to-case ratio of 2.2 per 1,000 cases). Forty-nine percent of deaths were among children younger than 5 years of age. Ninety percent of fatal cases occurred among persons with no history of vaccination. Sixty-four deaths were reported in 1990, the largest annual number of deaths from measles since 1971.
The most important cause of the measles resurgence of 1989–1991 was low vaccination coverage. Measles vaccine coverage was low in many cities, including some that experienced large outbreaks among preschool-aged children throughout the early to mid-1980s. Surveys in areas experiencing outbreaks among preschool-aged children indicated that as few as 50% of children had been vaccinated against measles by their second birthday, and that black and Hispanic children were less likely to be age-appropriately vaccinated than were white children.
In addition, measles susceptibility of infants younger than 1 year of age may have increased. During the 1989–1991 measles resurgence, incidence rates for infants were more than twice as high as those in any other age group. The mothers of many infants who developed measles were young, and their measles immunity was most often due to vaccination rather than infection with wild virus. As a result, a smaller amount of antibody was transferred across the placenta to the fetus, compared with antibody transfer from mothers who had higher antibody titers resulting from wild-virus infection. The lower quantity of antibody resulted in immunity that waned more rapidly, making infants susceptible at a younger age than in the past.
The increase in measles in 1989–1991 was not limited to the United States. Large outbreaks of measles were reported by many other countries of North and Central America, including Canada, El Salvador, Guatemala, Honduras, Jamaica, Mexico, and Nicaragua.
Measles Since 1993
Measles 1993-2011
- Endemic transmission interrupted
- Record low annual total in 2004 (37 total cases)
- Many cases among adults
- Most cases imported or linked to importation
- Most persons with measles were unvaccinated or unknown vaccination status
- In 2011, CDC reported 16 outbreaks of measles and 220 measles cases, most of which were imported cases in unvaccinated persons
Reported cases of measles declined rapidly after the 1989–1991 resurgence. This decline was due primarily to intensive efforts to vaccinate preschool-aged children. Measles vaccination levels among 2-year-old children increased from 70% in 1990 to 91% in 1997.
Since 1993, fewer than 500 cases have been reported annually, and fewer than 200 cases per year have been reported since 1997. A record low annual total of 37 cases was reported in 2004. Available epidemiologic and virologic data indicate that measles transmission in the United States has been interrupted. The majority of cases are now imported from other countries or linked to imported cases. Most imported cases originate in Asia and Europe and occur both among U.S. citizens traveling abroad and persons visiting the United States from other countries. An aggressive measles vaccination program by the Pan American Health Organization (PAHO) has resulted in record low measles incidence in Latin America and the Caribbean, and the interruption of indigenous measles transmission in the Americas. Measles elimination from the Americas was achieved in 2002 and has been sustained since then, with only imported and importation-related measles cases occuring in the region.
Since the mid-1990s, no age group has predominated among reported cases of measles. Relative to earlier decades, an increased proportion of cases now occur among adults. In 1973, persons 20 years of age and older accounted for only about 3% of cases. In 1994, adults accounted for 24% of cases, and in 2001, for 48% of all reported cases.
The size and makeup of measles outbreaks has changed since the 1980s. Prior to 1989, the majority of outbreaks occurred among middle, high school and college student populations. As many as 95% of persons infected during these outbreaks had received one prior dose of measles vaccine. A second dose of measles vaccine was recommended for school-aged children in 1989, and all states now require two doses of measles vaccine for school-aged children. As a result, measles outbreaks in school settings are now uncommon.
In 2008 a total of 140 measles cases was reported, the largest annual total since 1996. Eighty nine percent of these cases were imported from or associated with importations from other countries, particularly countries in Europe where several outbreaks are ongoing. Persons younger than 20 years of age accounted for 76% of the cases; 91% were in persons who were unvaccinated (most because of personal or religious beliefs) or of unknown vaccination status. The increase in the number of cases of measles in 2008 was not a result of a greater number of imported measles cases. It was the result of more measles transmission after the virus was imported. The importation-associated cases occurred largely among school-aged children who were eligible for vaccination but whose parents chose not to have them vaccinated. Many of these children were home-schooled and not subject to school entry vaccination requirements.
In 2011, CDC reported 16 outbreaks of measles and 220 measles cases, most of which were imported cases in unvaccinated persons. Among the U.S. measles cases in persons 16 months through 19 years reported in 2011, 62% were in persons not vaccinated for a nonmedical reason.
See information about the clinical case definition, clinical classification and epidemiologic classification of measles.
Measles Vaccines
- 1963 - Live attenuated and inactivated “killed” vaccines
- 1965 - Live further attenuated vaccine
- 1967 - Killed vaccine withdrawn
- 1968 - Live further attenuated vaccine (Edmonston-Enders strain)
- 1971 - Licensure of combined measles-mumps-rubella vaccine
- 1989 - Two-dose schedule
- 2005 - Licensure of combined measles-mumps-rubella-varicella vaccine
Measles Vaccines
- Composition
- Live virus
- Efficacy
- 95% at 12 months of age
- 98% at 15 months of age
- Duration of Immunity
- lifelong
- Schedule
- 2 doses
- should be administered with mumps and rubella as MMR or with mumps, rubella and varicella as MMRV
- single-antigen measles vaccine not available in the United States
Measles Mumps Rubella (MMR) Vaccine Failure
- Measles, mumps, or rubella disease (or lack of immunity) in a previously vaccinated person
- 2%-5% of recipients do not respond to the first dose
- Caused by antibody, damaged vaccine, incorrect records
- Most persons with vaccine failure will respond to second dose
Measles (MMR) Vaccine Indications
- All children 12 months of age and older
- Susceptible adolescents and adults without documented evidence of immunity
Measles Vaccine
Measles virus was first isolated by John Enders in 1954. The first measles vaccines were licensed in 1963. In that year, both an inactivated (“killed”) and a live attenuated vaccine (Edmonston B strain) were licensed for use in the United States. The inactivated vaccine was withdrawn in 1967 because it did not protect against measles virus infection. Furthermore, recipients of inactivated measles vaccine frequently developed a unique syndrome, atypical measles, if they were infected with wild-type measles virus (see Atypical Measles, in the Complications section). The original Edmonston B vaccine was withdrawn in 1975 because of a relatively high frequency of fever and rash in recipients. A live, further attenuated vaccine (Schwarz strain) was first introduced in 1965 but also is no longer used in the United States. Another live, further attenuated strain vaccine (Edmonston-Enders strain) was licensed in 1968. These further attenuated vaccines caused fewer reactions than the original Edmonston B vaccine.
Characteristics
The only measles virus vaccine now available in the United States is a live, more attenuated Edmonston-Enders strain (formerly called “Moraten”). The vaccine is available combined with mumps and rubella vaccines as MMR, or combined with mumps, rubella, and varicella vaccine as MMRV (ProQuad). The Advisory Committee on Immunization Practices (ACIP) recommends that MMR be used when any of the individual components is indicated. Single-antigen measles vaccine is not available in the United States.
Measles vaccine is prepared in chick embryo fibroblast tissue culture. MMR and MMRV are supplied as a lyophylized (freeze-dried) powder and are reconstituted with sterile, preservative-free water. The vaccines contain small amounts of human albumin, neomycin, sorbitol, and gelatin.
Immunogenicity and Vaccine Efficacy
Measles vaccine produces an inapparent or mild, noncommunicable infection. Measles antibodies develop in approximately 95% of children vaccinated at 12 months of age and 98% of children vaccinated at 15 months of age. Seroconversion rates are similar for single-antigen measles vaccine, MMR, and MMRV. Approximately 2%–5% of children who receive only one dose of MMR vaccine fail to respond to it (i.e., primary vaccine failure). MMR vaccine failure may occur because of passive antibody in the vaccine recipient, damaged vaccine, incorrect records, or possibly other reasons. Most persons who fail to respond to the first dose will respond to a second dose. Studies indicate that more than 99% of persons who receive two doses of measles vaccine (with the first dose administered no earlier than the first birthday) develop serologic evidence of measles immunity.
Although the titer of vaccine-induced antibodies is lower than that following natural disease, both serologic and epidemiologic evidence indicate that vaccine-induced immunity appears to be long-term and probably lifelong in most persons. Most vaccinated persons who appear to lose antibody show an anamnestic immune response upon revaccination, indicating that they are probably still immune. Although revaccination can increase antibody titer in some persons, available data indicate that the increased titer may not be sustained. Some studies indicate that secondary vaccine failure (waning immunity) may occur after successful vaccination, but this appears to occur rarely and to play only a minor role in measles transmission and outbreaks.
Vaccination Schedule and Use
MMR Vaccine
- First dose of MMR at 12-15 months
- 12 months is the minimum age
- MMR given before 12 months should not be counted as a valid dose
- Revaccinate at 12 months of age or older
Second Dose of Measles Vaccine
- Second dose of MMR at 4-6 years
- Second dose may be given any time at least 4 weeks after the first dose
- Intended to produce measles immunity in persons who failed to respond to the first dose (primary vaccine failure)
- May boost antibody titers in some persons
MMR and MMRV Vaccine
- For the first dose of measles, mumps, rubella, and varicella vaccines either MMR and varicella vaccines or MMRV vaccine can be used
- Providers should discuss the benefits and risks of both vaccination options with the parents or caregivers
- Unless the parent or caregiver expresses preference for MMRV, CDC recommends using MMR and varicella vaccines for the first dose
- Providers who face barriers to clearly communicating benefits and risks for any reason, such as language barriers, should administer MMR and varicella vaccines separately
- For the second dose at any age, use of MMRV vaccine generally is preferred over separate injections of MMR and varicella vaccines
Two doses of measles-containing vaccine, as combination MMR, separated by at least 4 weeks, are routinely recommended for all children 12 months of age or older. All persons born during or after 1957 should have documentation of at least one dose of MMR or other evidence of measles immunity. Certain adolescents and adults should receive two doses of MMR.
The first dose of MMR should be given on or after the first birthday. Any dose of measles-containing vaccine given before 12 months of age should not be counted as part of the series. Children vaccinated with measles-containing vaccine before 12 months of age should be revaccinated with two doses of MMR vaccine, the first of which should be administered when the child is at least 12 months of age.
A second dose of MMR is recommended to produce immunity in those who failed to respond to the first dose. The second dose of MMR vaccine should routinely be given at age 4–6 years, before a child enters kindergarten or first grade. The recommended visit at age 11 or 12 years can serve as a catch-up opportunity to verify vaccination status and administer MMR vaccine to those children who have not yet received two doses of MMR.
The second dose of MMR may be administered as soon as 4 weeks (28 days) after the first dose. Children who have already received two doses of MMR vaccine at least 4 weeks apart, with the first dose administered no earlier than the first birthday, do not need an additional dose when they enter school. Children without documentation of adequate vaccination against measles, mumps, and rubella or other acceptable evidence of immunity to these diseases when they enter school should be admitted after receipt of the first dose of MMR. A second dose should be administered as soon as possible, but no less than 4 weeks after the first dose.
Only doses of vaccine with written documentation of the date of receipt should be accepted as valid. Self-reported doses or a parental report of vaccination is not considered adequate documentation. A healthcare provider should not provide an immunization record for a patient unless that healthcare provider has administered the vaccine or has seen a record that documents vaccination. Persons who lack adequate documentation of vaccination or other acceptable evidence of immunity should be vaccinated. Vaccination status and receipt of all vaccinations should be documented in the patient’s permanent medical record and in a vaccination record held by the individual.
MMRV is approved by the Food and Drug Administration for children 12 months through 12 years of age (that is, until the 13th birthday). MMRV should not be administered to persons 13 years of age or older.
For the first dose of MMR and varicella vaccine at age 12 through 47 months, either MMR vaccine and varicella vaccine or MMRV vaccine may be used. Providers who are considering administering MMRV vaccine should discuss the benefits and risks of both vaccination options with the parents or caregivers. Unless the parent or caregiver expresses a preference for MMRV vaccine, CDC recommends that MMR vaccine and varicella vaccine should be administered at separate sites for the first dose in this age group. See “Adverse Reactions” for more information. For the second dose of MMR and varicella vaccine at any age (15 months through 12 years) and for the first dose at 48 months of age or older, use of MMRV vaccine generally is preferred over separate injections of its equivalent component vaccines (i.e., MMR vaccine and varicella vaccine).
Vaccination of Adults
Adults at Increased Risk of Measles
- College students
- Persons working in medical facilities
- International travelers
Adults born in 1957 or later who do not have a medical contraindication should receive at least one dose of MMR vaccine unless they have documentation of vaccination with at least one dose of measles-, mumps- and rubella-containing vaccine or other acceptable evidence of immunity to these three diseases. With the exception of women who might become pregnant (see Rubella chapter) and persons who work in medical facilities, birth before 1957 generally can be considered acceptable evidence of immunity to measles, mumps, and rubella.
Certain groups of adults may be at increased risk for exposure to measles and should receive special consideration for vaccination. These include persons attending colleges and other post-high school educational institutions, persons working in medical facilities, and international travelers.
Colleges and other post-high school educational institutions are potential high-risk areas for measles, mumps, and rubella transmission because of large concentrations of susceptible persons. Prematriculation vaccination requirements for measles immunity have been shown to significantly decrease the risk of measles outbreaks on college campuses where they are implemented and enforced. Colleges, universities, technical and vocational schools, and other institutions for post-high school education should require documentation of two doses of MMR vaccine or other acceptable evidence of measles, mumps, and rubella immunity before entry.
Students who have no documentation of live measles, mumps, or rubella vaccination or other acceptable evidence of measles, mumps, and rubella immunity at the time of enrollment should be admitted to classes only after receiving the first dose of MMR. A second dose of MMR should be administered no less than 4 weeks (28 days) later. Students with evidence of prior receipt of only one dose of MMR or other measles-containing vaccine on or after their first birthday should receive a second dose of MMR, provided at least 4 weeks have elapsed since their previous dose.
Measles Immunity in Healthcare Personnel
- All persons who work within medical facilities should have evidence of immunity to measles
Persons who work in medical facilities are at higher risk for exposure to measles than the general population. All persons who work within medical facilities should have evidence of immunity to measles, mumps, and rubella. Because any healthcare personnel (i.e., medical or nonmedical, paid or volunteer, full time or part time, student or nonstudent, with or without patient-care responsibilities) who lack evidence of immunity to measles or rubella can contract and transmit these diseases, all medical facilities (i.e., inpatient and outpatient, private and public) should ensure measles and rubella immunity among those who work within their facilities.
Adequate vaccination for measles, mumps, and rubella for healthcare personnel born during or after 1957 consists of two doses of a live measles- and mumps-containing vaccine and at least one dose of a live rubella-containing vaccine. Healthcare personnel needing a second dose of measles-containing vaccine should be revaccinated at least 4 weeks after their first dose.
For unvaccinated personnel born before 1957 who lack laboratory evidence of measles, mumps and/or rubella immunity or laboratory confirmation of disease, healthcare facilities should consider vaccinating personnel with two doses of MMR vaccine at the appropriate interval (for measles and mumps) and one dose of MMR vaccine (for rubella), respectively. For unvaccinated personnel born before 1957 who lack laboratory evidence of measles, mumps and/or rubella immunity or laboratory confirmation of disease, healthcare facilities should recommend two doses of MMR vaccine during an outbreak of measles or mumps and one dose during an outbreak of rubella.
Serologic testing does not need to be done before vaccinating for measles and rubella unless the healthcare facility considers it cost-effective. Serologic testing is appropriate only if tracking systems are used to ensure that tested persons who are identified as susceptible are subsequently vaccinated in a timely manner. Serologic testing for immunity to measles and rubella is not necessary for persons documented to be appropriately vaccinated or who have other acceptable evidence of immunity. If the return and timely vaccination of those screened cannot be assured, serologic testing before vaccination should not be done.
Persons who travel outside the United States are at increased risk of exposure to measles. Measles is endemic or epidemic in many countries throughout the world. Although proof of immunization is not required for entry into the United States or any other country, persons traveling or living abroad should have evidence of measles immunity. Adequate vaccination of persons who travel outside the United States is two doses of MMR.
Revaccination
Measles Vaccine Indications for Revaccination
- Vaccinated before the first birthday
- Vaccinated with killed measles vaccine (KMV)
- Vaccinated from 1963 through 1967 with an unknown type of vaccine
- Vaccinated with IG in addition to a further attenuated strain or vaccine of unknown type
Revaccination is recommended for certain persons. The following groups should be considered unvaccinated and should receive at least one dose of measles vaccine: persons 1) vaccinated before the first birthday, 2) vaccinated with killed measles vaccine (KMV), 3) vaccinated from 1963 through 1967 with an unknown type of vaccine, or 4) vaccinated with immune globulin (IG) in addition to a further attenuated strain or vaccine of unknown type. (Revaccination is not necessary if IG was given with Edmonston B vaccine).
Postexposure Prophylaxis
Live measles vaccine provides permanent protection and may prevent disease if given within 72 hours of exposure. IG may prevent or modify disease and provide temporary protection if given within 6 days of exposure. The dose is 0.5 mL/kg body weight, with a maximum of 15 mL intramuscularly and the recommended dose of IG given intravenously is 400mg/kg. IG may be especially indicated for susceptible household contacts of measles patients, particularly contacts younger than 1 year of age (for whom the risk of complications is highest). If the child is 12 months of age or older, live measles vaccine should be given about 5 months later when the passive measles antibodies have waned. IG should not be used to control measles outbreaks. Guidance for outbreak control for measles can be found in the Manual for the Surveillance of Vaccine-Preventable Diseases.
Contraindications and Precautions to Vaccination
MMR Vaccine Contraindication and Precautions
- History of anaphylactic reactions to neomycin
- History of severe allergic reaction to any component of the vaccine
- Pregnancy
- Immunosuppression
- Moderate or severe acute illness
- Recent blood product
- Personal or family (i.e., sibling or parent) history of seizures of any etiology (MMRV only)
Measles and Mumps Vaccines and Egg Allergy
- Measles and mumps viruses grown in chick embryo fibroblast culture
- Studies have demonstrated safety of MMR in egg-allergic children
- Vaccinate without testing
Contraindications for MMR and MMRV vaccines include history of anaphylactic reactions to neomycin, history of severe allergic reaction to any component of the vaccine, pregnancy, and immunosuppression.
In the past, persons with a history of anaphylactic reactions following egg ingestion were considered to be at increased risk for serious reactions after receipt of measles- or mumps-containing vaccines, which are produced in chick embryo fibroblasts. However, data suggest that anaphylactic reactions to measles- and mumps-containing vaccines are not associated with hypersensitivity to egg antigens but to other components of the vaccines (such as gelatin). The risk for serious allergic reactions following receipt of these vaccines by egg-allergic persons is extremely low, and skin-testing with vaccine is not predictive of allergic reaction to vaccination. Therefore, MMR may be administered to egg-allergic children without prior routine skin testing or the use of special protocols.
MMR vaccine does not contain penicillin. A history of penicillin allergy is not a contraindication to vaccination with MMR or any other U.S. vaccine.
Women known to be pregnant should not receive measles vaccine. Pregnancy should be avoided for 4 weeks following MMR vaccine. Close contact with a pregnant woman is NOT a contraindication to MMR vaccination of the contact. Breastfeeding is NOT a contraindication to vaccination of either the woman or the breastfeeding child.
Replication of vaccine viruses can be prolonged in persons who are immunosuppressed or immunodeficient. Severe immunosuppression can be due to a variety of conditions, including congenital immunodeficiency, HIV infection, leukemia, lymphoma, generalized malignancy, or therapy with alkylating agents, antimetabolites, radiation, or large doses of corticosteroids. Evidence based on case reports has linked measles vaccine virus infection to subsequent death in at least six severely immunocompromised persons. For this reason, patients who are severely immunocompromised for any reason should not be given MMR vaccine. Healthy susceptible close contacts of severely immunocompromised persons should be vaccinated.
In general, persons receiving large daily doses of corticosteroids (2 mg/kg or more per day, or 20 mg or more per day of prednisone) for 14 days or more should not receive MMR vaccine because of concern about vaccine safety. MMR and its component vaccines should be avoided for at least 1 month after cessation of high-dose therapy. Persons receiving low-dose or short-course (less than 14 days) therapy, alternate-day treatment, maintenance physiologic doses, or topical, aerosol, intra-articular, bursal, or tendon injections may be vaccinated. Although persons receiving high doses of systemic corticosteroids daily or on alternate days for less than 14 days generally can receive MMR or its component vaccines immediately after cessation of treatment, some experts prefer waiting until 2 weeks after completion of therapy.
Patients with leukemia in remission who have not received chemotherapy for at least 3 months may receive MMR or its component vaccines.
Measles Vaccine and HIV Infection
- MMR recommended for persons who do not have evidence of current severe immunosuppression
- Prevaccination HIV testing not recommended
- MMRV not for use in persons with HIV infection
Measles disease can be severe in persons with HIV infection. Available data indicate that vaccination with MMR has not been associated with severe or unusual adverse reactions in HIV-infected persons without evidence of severe immunosuppression, although antibody responses have been variable. MMR vaccine is recommended for all persons 12 months of age or older with HIV infection who do not have evidence of current severe immunosuppression [absence of severe immunosuppression is defined as CD4 percentages greater than or equal to 15% for 6 months or longer for persons five years of age or younger; and CD4 percentages greater than or equal to 15% and CD4 count greater than or equal to 200 cells/mm3 for 6 months or longer for persons older than five years] or other current evidence of measles, rubella, and mumps immunity. Asymptomatic children do not need to be evaluated and tested for HIV infection before MMR or other measles-containing vaccines are administered. A theoretical risk of an increase (probably transient) in HIV viral load following MMR vaccination exists because such an effect has been observed with other vaccines. The clinical significance of such an increase is not known.
MMR and other measles-containing vaccines are not recommended for HIV-infected persons with evidence of severe immunosuppression. MMRV is not approved for and should not be administered to a person known to be infected with HIV.
Persons with moderate or severe acute illness should not be vaccinated until the patient has improved. This precaution is intended to prevent complicating the management of an ill patient with a potential vaccine adverse reaction, such as fever. Minor illness (e.g., otitis media, mild upper respiratory infections), concurrent antibiotic therapy, and exposure to or recovery from other illness are not contraindications to measles vaccination.
Receipt of antibody-containing blood products (e.g., immune globulin, whole blood or packed red blood cells, intravenous immune globulin) may interfere with seroconversion after measles vaccine. The length of time that such passively acquired antibody persists depends on the concentration and quantity of blood product received. For instance, it is recommended that vaccination be delayed for 3 months following receipt of immune globulin for prophylaxis of hepatitis A; a 7 to 11 month delay is recommended following administration of intravenous immune globulin, depending on the dose. For more information, see Chapter 2, General Recommendations on Immunization, and the table in Appendix A [32 pages].
Tuberculin Skin Testing (TST)* and Measles Vaccine
- Apply TST at same visit as MMR
- Delay TST at least 4 weeks if MMR given first
- Apply TST first and administer MMR when skin test read (least favored option because receipt of MMR is delayed)
*previously called PPD
Persons who have a history of thrombocytopenic purpura or thrombocytopenia (low platelet count) may be at increased risk for developing clinically significant thrombocytopenia after MMR vaccination. No deaths have been reported as a direct consequence of vaccine-induced thrombocytopenia. The decision to vaccinate with MMR depends on the benefits of immunity to measles, mumps, and rubella and the risks for recurrence or exacerbation of thrombocytopenia after vaccination or during natural infection with measles or rubella. The benefits of immunization are usually greater than the potential risks, and administration of MMR vaccine is justified because of the even greater risk for thrombocytopenia after measles or rubella disease. However, deferring a subsequent dose of MMR vaccine may be prudent if the previous episode of thrombocytopenia occurred within 6 weeks after the previous dose of the vaccine. Serologic evidence of measles immunity in such persons may be sought in lieu of MMR vaccination.
A personal or family (i.e., sibling or parent) history of seizures of any etiology is a precaution for MMRV vaccination. Studies suggest that children who have a personal or family history of febrile seizures or family history of epilepsy are at increased risk for febrile seizures compared with children without such histories. Children with a personal or family history of seizures of any etiology generally should be vaccinated with MMR vaccine and varicella vaccine at separate sites because the risks for using MMRV vaccine in these children generally outweigh the benefits.
Tuberculin skin testing (TST) is not a prerequisite for vaccination with MMR or other measles-containing vaccine. TST has no effect on the response to MMR vaccination. However, measles vaccine (and possibly mumps, rubella, and varicella vaccines) may transiently suppress the response to TST in a person infected with Mycobacterium tuberculosis. If tuberculin skin testing is needed at the same time as administration of measles-containing vaccine, TST and vaccine can be administered at the same visit. Simultaneously administering TST and measles-containing vaccine does not interfere with reading the TST result at 48–72 hours and ensures that the person has received measles vaccine. If the measles-containing vaccine has been administered recently, TST screening should be delayed at least 4 weeks after vaccination. A delay in administering TST will remove the concern of any theoretical suppression of TST reactivity from the vaccine. TST screening can be performed and read before administering the measles-containing vaccine. This option is the least favored because it will delay receipt of the vaccine.
Adverse Events Following Vaccination
MMR Adverse Events
- Arthralgias (susceptible women)
- 25%
- Rash, pruritis, purpura
- not common
MMR Vaccine and Autism
- To date there is no convincing evidence that any vaccine causes autism or autism spectrum disorder
Arthralgias and other joint symptoms are reported in up to 25% of susceptible adult women given MMR vaccine. This adverse event is associated with the rubella component (see Rubella chapter for more details).
Allergic reactions including rash, pruritus, and purpura have been temporally associated with mumps vaccination, but these are not common and usually mild and of brief duration.
To date there is no convincing evidence that any vaccine causes autism or autism spectrum disorder. Concern has been raised about a possible relation between MMR vaccine and autism by some parents of children with autism. Symptoms of autism are often noticed by parents during the second year of life, and may follow administration of MMR by weeks or months. Two independent nongovernmental groups, the Institute of Medicine (IOM) and the American Academy of Pediatrics (AAP), have reviewed the evidence regarding a potential link between autism and MMR vaccine. Both groups independently concluded that available evidence does not support an association, and that the United States should continue its current MMR vaccination policy. Additional research on the causes of autism is needed.
Adverse Reactions Following Vaccination
MMR Adverse Reactions
- Fever
- 5%-15%
- Rash
- 5%
- Thrombocytopenia
- 1/30,000-40,000 doses
- Lymphadenopathy
- rare
- Allergic reactions
- rare
Adverse reactions following measles vaccine (except allergic reactions) may be caused by replication of measles vaccine virus with subsequent mild illness. These events occur 5 to 12 days postvaccination and only in persons who are susceptible to infection. There is no evidence of increased risk of adverse reactions following MMR vaccination in persons who are already immune to the diseases.
Fever is the most common adverse reaction following MMR vaccination. Although measles, mumps, and rubella vaccines may cause fever after vaccination, the measles component of MMR vaccine is most often associated with fever. After MMR vaccination, 5% to 15% of susceptible persons develop a temperature of 103°F (39.4°C) or higher, usually occurring 7 to 12 days after vaccination and generally lasting 1 or 2 days. Most persons with fever are otherwise asymptomatic.
In MMRV vaccine prelicensure studies conducted among children 12–23 months of age, fever (reported as abnormal or elevated 102°F or higher oral equivalent) was observed 5-12 days after vaccination in 21.5% of MMRV vaccine recipients compared with 14.9% of MMR vaccine and varicella vaccine recipients. Two postlicensure studies indicated that among children 12–23 months of age, one additional febrile seizure occurred 5–12 days after vaccination per 2,300–2,600 children who had received the first dose of MMRV vaccine, compared with children who had received the first dose of MMR vaccine and varicella vaccine administered as separate injections at the same visit. Data from postlicensure studies do not suggest that children 4–6 years of age who received the second dose of MMRV vaccine had an increased risk for febrile seizures after vaccination compared with children the same age who received MMR vaccine and varicella vaccine administered as separate injections at the same visit.
MMRV and Febrile Seizure
- Among children 12-23 months of age one additional febrile seizure occurred 5-12 days after vaccination per 2,300–2,600 children compared to children who received the first dose of MMR and varicella vaccine separately
- Data do not suggest that children 4-6 years of age who received the second dose had an increased risk
Measles- and rubella-containing vaccines, including MMR, may cause a transient rash. Rashes, usually appearing 7 to 10 days after MMR or measles vaccination, have been reported in approximately 5% of vaccinees.
Rarely, MMR vaccine may cause thrombocytopenia within 2 months after vaccination. Estimates of the frequency of clinically apparent thrombocytopenia from Europe are one case per 30,000–40,000 vaccinated susceptible persons, with a temporal clustering of cases occurring 2 to 3 weeks after vaccination. The clinical course of these cases was usually transient and benign, although hemorrhage occurred rarely. The risk for thrombocytopenia during rubella or measles infection is much greater than the risk after vaccination. Based on case reports, the risk for MMR-associated thrombocytopenia may be higher for persons who have previously had immune thrombocytopenic purpura, particularly for those who had thrombocytopenic purpura after an earlier dose of MMR vaccine.
Transient lymphadenopathy sometimes occurs following receipt of MMR or other rubella-containing vaccine, and parotitis has been reported rarely following receipt of MMR or other mumps-containing vaccine.
Allergic reactions following the administration of MMR or any of its component vaccines are rare. Most of these reactions are minor and consist of a wheal and flare or urticaria at the injection site. Immediate, anaphylactic reactions to MMR or its component vaccines are extremely rare.
Vaccine Storage and Handling
MMR vaccine can be stored either in the freezer or the refrigerator and should be protected from light at all times. MMRV vaccine should be stored frozen between -58°F and +5°F (-50°C and -15°C). When MMR vaccine is stored in the freezer, the temperature should be the same as that required for MMRV, between -58°F and +5°F (-50°C and -15°C). Storing MMR in the freezer with MMRV may help prevent inadvertent storage of MMRV in the refrigerator.
Manufacturer package inserts contain additional information. For complete information on best practices and recommendations please refer to CDC’s Vaccine Storage and Handling Toolkit [4.33 MB, 109 pages].
Acknowledgment
The editors thank Drs. Gregory Wallace, and Zaney Leroy, CDC for their assistance in updating this chapter.
Selected References
- American Academy of Pediatrics. Measles. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:444–55.
- Atkinson WL, Orenstein WA, Krugman S. The resurgence of measles in the United States, 1989–1990. Ann Rev Med 1992;43:451–63.
- Bellini WJ, Rota PA. Genetic diversity of wild-type measles viruses: implications for global measles elimination programs. Emerg Infect Dis 1998;4:29–35.
- Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis 2005;192:1686–93.
- CDC. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8):1–57.
- CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2013;62(No. 4):1-34.
- CDC. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-3):1–12.
- CDC. Immunization of health-care personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(RR-7):
1-45. - CDC. Update: Measles — United States, January–July 2008. MMWR 2008;57:893–6.
- CDC. Global measles mortality, 2000–2008. MMWR 2009;58:1321–6.
- Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis 2009;48:456–61.
- Halsey NA, Hyman SL, Conference Writing Panel. Measles-mumps-rubella vaccine and autistic spectrum disorder: report from the New Challenges in Childhood Immunizations Conference convened in Oak Brook, IL, June 12–13, 2000. Pediatrics 2001;107(5).
- Institute of Medicine. Institute of Medicine immunization safety review: vaccines and autism. Washington DC: National Academy Press, 2004.
- Sugerman DE, Barskey AE, Delea MG et al. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics 2010;125:747-52.
- Vitek CR, Aduddel, M, Brinton MJ. Increased protection during a measles outbreak of children previously vaccinated with a second dose of measles-mumps-rubella vaccine. Pediatr Infect Dis J 1999;18:620–3.
- Page last reviewed: November 15, 2016
- Page last updated: July 24, 2015
- Content source:


 ShareCompartir
ShareCompartir