Vaccine Administration
Printer friendly version [28 pages]
On this Page
Proper vaccine administration is a critical component of a successful immunization program. It is a key part of ensuring that vaccination is as safe and effective as possible. This chapter provides best practice guidance for vaccine administration. The guidance should be used in conjunction with professional standards for medication administration and guidance from the vaccine manufacturer.
Vaccine Administration
- Key to ensuring vaccination is as safe and effective as possible
- Incorporate
- professional standards for medication administration
- manufacturer’s vaccine-specific guidelines
- evidence-based safe injection practices on CDC’s Injection Safety Information for Providers webpage
Staff Training and Education
- Before administering vaccines, all personnell who administer vaccines should
- receive competency-based training
- validate knowledge and skills
- Integrate training into
- new staff orientation
- annual education requirements
- when vaccine administration recommendations are updated
- when new vaccines are added to the inventory
The foundation of medication administration is application of the “Rights of Medication Administration.” These rights should be applied to each encounter when vaccines are administered. These rights include the:
- Right patient
- Right vaccine and diluent (when applicable)
- Right time (including the correct age and interval, as well as before the product expiration time/date)
- Right dosage
- Right route (including the correct needle gauge and length and technique)
- Right site
- Right documentation
Vaccine providers should also incorporate the evidence-based safe injection practices, outlined on CDC’s Injection Safety Information for Providers webpage.
Staff Training and Education
Improper administration of vaccines may result in injuries or prevent the vaccines from providing optimal protection. All personnel who will administer vaccines should receive comprehensive, competency-based training regarding vaccine administration policies and procedures before administering vaccines. Providers need to validate staff’s knowledge and skills regarding vaccine administration with a skills checklist. See the Skills Checklist for Immunization [2 pages] for an example. Competency-based training should be integrated into existing staff education programs such as new staff orientation and annual education requirements. Staff should receive ongoing education, such as whenever vaccine administration recommendations are updated, or when new vaccines are added to the facility’s inventory, to maintain staff competency. Accountability checks should be put in place to ensure policies and procedures are followed. Trainings should also be offered to temporary personnel who may be filling in on days when the facility is short staffed or helping during peak times such as flu season. Evidence-based injection safety information and educational programs for healthcare personnel are available on the CDC Injection Safety website. In addition, the Immunization Action Coalition (IAC) offers web-based educational programs and job aids. See IAC resources for administering vaccines.
Patient Care Before Administering Vaccine
Patient Care Before Administering Vaccines
- Obtain complete immunization history at every healthcare visit
- accept only written, dated records (exception influenza and PPSV23 self-report)
- use recommended schedule to determine vaccines needed based on age, medical condition, and risk factors
- Screen for contraindications and precautions prior to administering any vaccine(s)
- Discuss vaccine benefits and risks and vaccine-preventable disease risks using VISs and other reliable resources
- Provide after-care instructions
All immunization providers should be knowledgeable regarding appropriate strategies to prepare and care for patients whenever vaccines will be administered.
Immunization Assessment
The patient’s immunization history should be reviewed at every healthcare visit. When the patient arrives, providers should obtain a complete immunization history, and compare the patient’s immunization record to the medical record and immunization information system or registry data, if available. Use the current immunization schedule based on the age of the patient to determine all recommended vaccines that are needed. Assess for all routinely recommended vaccines as well as any vaccines that are indicated based on health status, occupation, or other risk factors. If a documented immunization history is not available, administer the vaccines that are indicated based on the person’s age, medical condition and other risk factors. With the exception of influenza and pneumococcal polysaccharide vaccine (PPSV23), providers should only accept written, dated records as evidence of vaccination; self-reported doses of influenza vaccine and PPSV23 are acceptable. This prevents missing an opportunity to vaccinate while the patient or parent searches for the immunization record.
Screening for Contraindications and Precautions
Patients and their family members count on providers and their staff to administer vaccines safely. Screening for contraindications and precautions can prevent adverse events following vaccination. All patients should be screened for contraindications and precautions prior to administering any vaccine, even if the patient has previously received that vaccine. The patient’s status may change from one visit to the next or recommendations regarding contraindications and precautions may have changed. Staff should be knowledgeable of all possible contraindications and precautions to vaccination and only valid contraindications should be followed. See more information about contraindications and precautions.
Screening for contraindications and precautions should be included in vaccine administration procedures. Using a standardized screening tool helps staff assess patients correctly and consistently. Many state immunization programs and other organizations have developed standardized screening tools. Two examples are Screening Checklist for Contraindications to Vaccines for Children and Teens [2 pages] and Screening Checklist for Contraindications to Vaccines for Adults [2 pages]. In addition, both screening checklists are available in other languages. To save time, some facilities ask patients to answer screening questions prior to seeing the provider, such as electronically via an electronic healthcare portal or with a paper copy and pen while in the waiting or exam room.
Patient or Parent Education including Vaccine Safety & Risk Communication
Research shows that parents want clear, consistent information from multiple sources they consider credible. Many of today’s parents do not know very much about vaccine-preventable diseases, and therefore do not understand vaccines’ disease-protection benefits. They often cite the Internet as the source of vaccine information. However, some of the information available online is not accurate and conflicting. It can be difficult for a parent to know which sites to believe. Therefore, parents may turn to their most trusted information source of vaccine information: their child’s doctor or nurse. Healthcare professionals need to be ready to provide parents with timely and transparent information about vaccine benefits and risks.
Establishing an open dialogue promotes a safe, trust-building environment in which individuals can freely evaluate information, discuss vaccine concerns and make informed decisions regarding immunizations. Not all parents want the same level of medical or scientific information about vaccines. Healthcare professionals are encouraged to assess the level of information that each parent wants and provide clear and transparent information. Research shows that a provider’s recommendation for vaccination is a powerful motivator.
Immunization providers may be asked about many topics, including vaccine-preventable diseases, specific vaccines, the immunization schedule, and vaccine safety issues. Fortunately, there are many resources available to help providers stay up-to-date on all of these vaccine-related issues.
Vaccine Information Statements (VISs) are information sheets produced by the Centers for Disease Control and Prevention (CDC) that explain to vaccine recipients, their parents, or their legal representatives both the benefits and risks of a vaccine. Federal law requires that VISs be handed out whenever vaccinations routinely recommended for children are administered, but CDC encourages the use of ALL VISs, whether the vaccine is covered by the law or not. The VIS should be given every time a dose of vaccine is administered, even if the patient has received the vaccine and a VIS in the past. VISs can be provided at the same time as the screening questionnaire, while the patient is waiting to be seen. They include information that may help the patient or parent respond to the screening questions. In addition to traditional paper copies, VISs are increasingly available in electronic formats that can read on smart phones and other devices.
Providers can also use the CDC website titled, Provider Resources for Vaccine Conversations with Parents, to talk to parents of infants and young children. The materials available on this website are based on formative, mixed methods research, informed by risk communication principles, and reviewed annually by subject matter experts. In addition, all fact sheets are co-branded with the American Academy of Pediatrics and the American Academy of Family Physicians. In addition, healthcare professionals may find the CDC resource, Tips and Time-savers for Talking with Parents about HPV Vaccine [1 page], helpful when talking with parents of adolescents.
A best practice strategy is to allow time for questions and discussion of after-care instructions with patients or parents/guardians before the vaccines are administered. This allows the parent to comfort the child immediately after the injection. After-care instructions should include information and strategies for dealing with side effects such as injection site pain, fever, fussiness (infants especially) and for determining when medical attention should be sought. An age-appropriate dose of a non-aspirin-containing pain reliever may be considered to decrease discomfort and fever after vaccination. The prophylactic use of antipyretics before or at the time of vaccination is not recommended. Examples of after-care instructional materials for parents and patients are After the Shots [1 page] and After Receiving Vaccines [1 page].
Patient Care During Vaccine Administration
Patient Care During Vaccine Administration
- Consider patient’s age and stage of development
- Encourage participation of parent/guardian and patient
- Use simple strategies to ease vaccination process
- positive attitude
- soft, calm voice
- eye contact
- explain why the vaccine is needed
- honest about what to expect
Positioning and Comforting Restraint
- Encourage parent/guardian to hold child
- Sitting, rather than lying down
- Be aware of syncope (fainting)
- have patient seated or lying down during vaccination
- be aware of symptoms that precede syncope
- if patient faints, provide supportive care and protect patient from injury
- observe patient (seated or lying down) for at least 15 minutes after vaccination
Patients should be prepared for vaccination with consideration for their age and stage of development. Parents/guardians and patients should be encouraged to take an active role before, during and after the administration of vaccines. Be There for Your Child During Shots [2 pages] is a handout for parents.
Vaccine safety concerns and the need for multiple injections have increased anxiety associated with immunizations for patients, parents and health-care personnel. Health-care providers need to display confidence and establish an environment that promotes a sense of security and trust. Everyone involved should work to provide immunizations in the safest and least stressful way possible. Simple strategies that can be used by both parents and providers to make receiving vaccines easier include:
- Displaying a positive attitude through facial expressions, body language, and comments
- Using a soft and calm tone of voice
- Making eye contact, even with small children
- Explaining why vaccines are needed (e.g., “this medicine will protect you from getting sick” or “this shot is a shield to protect your body against infection”)
- Being honest and explaining what to expect (e.g., do not say that “the injection won’t hurt”)
Positioning & Comforting Restraint
When determining patient positioning and restraint, consider the patient’s comfort, safety, age, activity level, and the site of administration. Parent participation has been shown to increase the child’s comfort. When vaccines are being administered to infants and small children, the parent/guardian should be encouraged to hold the child during administration. The parent/guardian should be instructed on how to help the child stay still so the vaccine can be administered safely. If the parent is uncomfortable, another person may assist or the patient may be positioned safely. Comforting Restraint for Immunizations [2 pages] outlines positioning techniques.
While definitive guidelines for positioning patients during vaccination have not been established, some recommendations have been suggested. Research supports the belief that children are less fearful and experience less pain when receiving an injection if they are sitting up rather than lying down. The exact mechanism behind this phenomenon is unknown; it may be that the child’s anxiety level is reduced, which in turn reduces the child’s perception of pain. Parents should be instructed to hold infants and children in a position comfortable for the child and parent, in which one or more limbs are exposed for injections. All providers who administer vaccines to older children, adolescents, and adults should be aware of the potential for syncope (fainting) after vaccination and the related risk of injury caused by falls. Clinicians should: (1) make sure the person who is being vaccinated is always seated or lying down; (2) be aware of symptoms that precede fainting (e.g. weakness, dizziness, pallor); and (3) provide supportive care and take appropriate measures to prevent injuries if such symptoms occur. The Advisory Committee on Immunization Practices (ACIP) also recommends that providers consider observing the patient (with patient seated or lying down) for 15 minutes after vaccination.
Procedural Pain Management
Procedural Pain Management
- Evidence-based strategies to ease pain
- breastfeeding
- sweet tasting solutions
- injection technique (aspiration may increase pain)
- order of injections (administer most painful vaccine last)
- tactile stimulation (rub/stroke near injection site prior to and during injection
- distraction
- topical anesthetic
Concern and anxiety about injections are common for all ages. Fear of injections and needlestick pain are often cited as reasons why children and adults, including health-care personnel, refuse vaccines. Immunizations are the most common source of iatrogenic pain and are administered repeatedly to children throughout infancy, childhood and adolescence. If not addressed, this pain can have long term effects such as pre-procedural anxiety, fear of needles and avoidance of healthcare behaviors through the lifetime. It has been estimated that up to 25% of adults have a fear of needles, with most fears developing in childhood. Decreasing pain associated with immunizations during childhood may help to prevent this distress and future healthcare avoidance behaviors.
Pain is a subjective phenomenon influenced by multiple factors, including an individual’s age, anxiety level, previous healthcare experiences, and culture. Although pain from immunizations is, to some extent, unavoidable, there are some things that parents and healthcare providers can do to help when children and adults need vaccines. Evidence-based strategies to ease the pain associated with the injection process include:
Breastfeeding
Breastfeeding has been demonstrated as a soothing measure for infants up to 12 months of age receiving injections. Several aspects of breastfeeding are thought to decrease pain, including holding the child, skin-to-skin contact, sweet-tasting milk and the act of sucking. Potential adverse events such as gagging or spitting up were not reported. Breastfeeding should occur before, during and after the administration of vaccines. Allow adequate time for the infant to latch onto the nipple properly. Bottle feeding with breast milk or formula should not be considered a substitute for breastfeeding for pain management.
Sweet tasting solutions
Sweet tasting liquids are an analgesic for infants up to 12 months of age. Sweetened liquids are recommended for infants who are not breastfed during vaccination. Several studies have demonstrated a reduction in crying after injections when young children (12 months or younger) ingest a small amount (a few drops to half a teaspoon) of a sugary solution prior to administration of the vaccine. Coughing and/or gagging may occur but infrequently (less than 5% of patients). Parents should be counseled that sweet tasting liquids should only be used for the management of pain associated with a procedure such as an injection.
Injection technique
Aspiration prior to injection and slowly injecting medication are practices that have not been evaluated scientifically. Aspiration was originally recommended for safety reasons and injecting medication slowly was thought to decrease pain from sudden distension of muscle tissue. Although aspiration is advocated by some experts, and most nurses are taught to aspirate before injection, there is no evidence that this procedure is necessary. The ACIP’s General Recommendations on Immunization document states that aspiration is not required before administering a vaccine. There are no reports of any person being injured because of failure to aspirate. In addition, the veins and arteries within reach of a needle in the anatomic areas recommended for vaccination are too small to allow an intravenous push of vaccine without blowing out the vessel. A 2007 study from Canada compared infants’ pain response using slow injection, aspiration, and slow withdrawal with another group using rapid injection, no aspiration, and rapid withdrawal. Based on behavioral and visual pain scales, the group that received the vaccine rapidly without aspiration experienced less pain. No adverse events were reported with either injection technique.
Order of injections
Frequently children and adults receive 2 or more injections at an immunization encounter. Some vaccines are associated with more pain than others. Because procedure pain can increase with each injection, the order the vaccines are administered may effect the overall pain response. Some vaccines cause a painful or stinging sensation when the injecting the vaccine; examples include measles, mumps and rubella (MMR) and human papillomavirus (HPV) vaccines. Injecting the most painful vaccine (e.g., MMR, PCV13, or HPV) last when multiple injections are being administered can decrease the pain associated with the injections.
Tactile Stimulation
Rubbing or stroking the skin near the injection site prior to and during the injection process with moderate intensity may decrease pain in older children (4 years and older) and adults. The mechanism for this is thought to be that the sensation of touch competes with the feeling of pain from the injection, and thereby results in less pain.
Distraction
Psychological interventions such as distraction in children have been demonstrated to be effective at reducing stress and the perception of pain during the injection process. Distraction is defined as using tactics which are intended to take the patient’s attention away from the procedure. Distraction can be led by the provider, child or parent. Certain types of parental behaviors (e.g., nonprocedural talk, suggestions on how to cope, humor) have been related to decreases in children’s distress and pain, whereas others (e.g., reassurances, apologies) have been related to increases in children’s distress and pain. Parents should be encouraged to use distraction methods and instructed in appropriate distraction techniques. Distraction can be accomplished through a variety of techniques (e.g., playing music, books, pretending to blow away the pain, deep breathing techniques).
Topical anesthetics
Topical analgesia may be applied to decrease pain at the injection site. These products (e.g., 5% lidocaine-prilocaine emulsion) should be used only for the ages recommended and as directed by the product manufacturer. Parents should be educated in the appropriate use of topical analgesics including the exact site(s) the medication should be applied. These analgesics often need to be applied before (20 to 60 minutes depending on the product) vaccine administration to be effective.
Following are other techniques used by some providers. There is insufficient evidence to recommend these techniques to relieve the pain associated with vaccine administration.
Dual administrators
Some providers suggest that having two individuals simultaneously administer vaccines at separate sites will decrease anxiety from anticipation of the next injection(s), while others believe this technique actually increases anxiety by making the child feel overpowered and vulnerable. At this time there is insufficient evidence to make a recommendation either for or against this technique.
Physical intervention “The 5 S’s”
A 2012 study found an intervention which included swaddling, holding the infant in a side/stomach position, shushing, swinging gently, and sucking provided decreased pain scores on a validated pain scale and decreased crying time for infants 2 and 4 months of age immediately following routine vaccinations.
Route of administration
As of March 2013, there are two FDA licensed vaccines (IPV and PPSV23) that can be administered by either the subcutaneous or intramuscular route. There is insufficient evidence to support one route (subcutaneous or intramuscular) versus the other as a way to reduce injection pain, in vaccines for which either route may be used. When more than one route is an option the number of injections and available sites may influence the vaccinator’s choice.
Infection Control
Infection Control
- Hand hygiene should be performed
- before vaccine preparation
- between patients
- any time hands become soiled
- Gloves are not required when administering vaccines unless the person administering the vaccine is likely to come into contact with potentially infectious body fluids or has open lesions on hands
- if gloves are worn, they should be changed and hand hygiene performed between patients
- Equipment disposal
- place used syringes and needles (do not cut, recap, or detach from syringe) in a puncture-resistant biohazard container
- dispose of empty or expired vaccine vials as medical waste
Healthcare providers should follow appropriate precautions to minimize the risks of spreading disease during the administration of vaccines.
Hand hygiene
Hand hygiene is critical to prevent the spread of illness and disease. Hand hygiene should be performed before vaccine preparation, between patients, and any time hands become soiled, e.g., diapering or cleansing excreta. Hands should be cleansed with a waterless alcohol-based hand rub or, when hands are visibly dirty or contaminated with blood or other body fluids, washed thoroughly with soap and water.
Gloves
Occupational Safety and Health Administration (OSHA) regulations do not require gloves to be worn when administering vaccines unless the person administering the vaccine is likely to come into contact with potentially infectious body fluids or has open lesions on the hands. If gloves are worn, they should be changed and hand hygiene performed between patients. Gloves will not prevent needlestick injuries. Any needlestick injury should be reported immediately to the site supervisor, with appropriate care and follow-up given as directed by local/state guidelines.
Equipment Disposal
Immediately after use, all used syringe/needle devices should be placed in biohazard containers that are closable, puncture-resistant, leakproof on sides and bottom and labeled or color-coded. This practice helps prevent accidental needlesticks and reuse. Used needles should not be recapped, cut, or detached from the syringes before disposal. Empty or expired vaccine vials are considered medical waste and should be disposed of according to state regulations. More information can be found at OSHA’s website.
Vaccine Preparation
Vaccine Preparation
- Equipment selection
- use a separate 1-mL or 3-mL sterile syringe for each injection
- OSHA requires safety- engineered injection devices to reduce risk of injury and disease transmission
- some syringes and needles are packaged with an expiration date
- select a separate sterile needle for each injection based on route, size of individual and injection technique
Proper vaccine handling and preparation is critical in maintaining the integrity of the vaccine during transfer from the manufacturer’s vial to the syringe and ultimately to the patient. Vaccines should be drawn up in a designated clean medication area that is not adjacent to areas where potentially contaminated items are placed. Multidose vials to be used for more than one patient should not be kept or accessed in the immediate patient treatment area. This is to prevent inadvertent contamination of the vial through direct or indirect contact with potentially contaminated surfaces or equipment that could then lead to infections in subsequent patients. If a multidose vial enters the immediate patient treatment area, it should be discarded after use. See other frequently asked questions on injection safety.
Equipment Selection
Syringe Selection
A separate needle and syringe should be used for each injection. A parenteral vaccine may be delivered in either a 1-mL or 3-mL syringe as long as the prescribed dosage is delivered. OSHA requires that safety-engineered injection devices (e.g., needle-shielding syringes or needle-free injectors) be used for injectable vaccination in all clinical settings to reduce risk for injury and disease transmission. Personnel who will be using these products should be involved in evaluation and selection of these products and should receive training with these devices before using them in the clinical area. Some syringes and needles are packaged with an expiration date. This can be a consideration when ordering injection supplies. Never administer medications from the same syringe to more than one patient, even if the needle is changed.
Needle Selection
Vaccine must reach the desired tissue site for optimal immune response to occur. Use of longer needles has been associated with less redness or swelling than occurs with shorter needles because of the injection into deeper muscle mass. Therefore, needle selection should be based on the prescribed route, size of the individual, and injection technique. A supply of needles in varying lengths appropriate for the facility’s patient population should be available to staff. Typically, vaccines are not highly viscous so a fine gauge needle (22- to 25-gauge) can be used. As with syringes, some needles are packaged with an expiration date. Check the expiration date on the needle and syringe packaging, if present. Do not use if the equipment has expired.
Inspecting Vaccine
Vaccine Preparation
- Inspect vaccine and diluent vial for damage or contamination
- Check the expiration date; never administer expired vaccine or diluent
- Reconstitute vaccine,
- if applicable, according
- to manufacturers guidelines just before administration using ONLY the manufacturers supplied diluent for that vaccine.
- Agitate vial to thoroughly mix vaccine
- Inspect vaccine for discoloration, precipitate or if it cannot be re-suspended
Each vaccine and diluent vial should be carefully inspected for damage or contamination prior to use. The expiration date printed on the vial or box should be checked. Vaccine can be used through the last day of the month indicated by the expiration date unless otherwise stated on the package labeling. The expiration date or time for some vaccines changes once the vaccine vial is opened or the vaccine is reconstituted. This information is available in the manufacturer’s product information. Regardless of expiration date, vaccine and diluent should only be used as long as they are normal in appearance and have been stored and handled properly. Expired vaccine or diluent should never be used.
Reconstitution
Several vaccines are supplied in a lyophilized (freeze-dried) form that requires reconstitution with a liquid diluent. Vaccines should be reconstituted according to manufacturer guidelines using only the specific diluent supplied by the manufacturer for that vaccine. Each diluent is specific to the corresponding vaccine in volume, sterility, pH, and chemical balance. If the wrong diluent is used, the vaccine dose is not valid and will need to be repeated using the correct diluent.
Reconstitute vaccine just before using. Inject all the diluent into the vaccine vial and agitate the vial to ensure thorough mixing (follow the specific instructions provided in the product information). Use all of the diluent supplied for a single dose and then draw up all of the vaccine after it is thoroughly reconstituted. Changing the needle between drawing vaccine from the vial and administering the vaccine is not necessary unless the needle is contaminated or damaged. For additional information on reconstituted vaccines, see Preparing Reconstituted Vaccine [1 page] and Vaccine with Diluents: How to use them [1 page].
Beyond Use Date (BUD)
Some vaccines should be used within a certain time frame after the first time a needle is inserted into a multidose vial (commonly referred to as “entering” the vial.) For other vaccines, this time frame is based on the date/time the vaccine was reconstituted. This time frame is called the “beyond use date” or BUD. The BUD is the date or time after which the vaccine should not be used. It may not be the same as the expiration date printed on the vial by the manufacturer. The BUD varies among vaccines and can be found in the package insert. Check the package insert to determine if the vaccine has a BUD and for the correct time frame (e.g., days, hours) the vaccine can be stored once the vial has been entered or has been reconstituted. Calculate the beyond use date using the time interval found in the vaccine’s package insert. Label the vaccine with the correct beyond use date/time and your initials. If the reconstituted vaccine is not used immediately, write the BUD and your initials on the label and store it properly. Refer to the CDC’s Vaccine Inventory Management [4.33 MB, 109 pages] for specific vaccine product information, including the beyond use dates.
Filling Syringes
Vaccine Preparation
- Filling syringe
- remove the vial dust cover and withdraw the vaccine according to standard medication preparation guidelines just prior to vaccination
- single-dose vials should only be used for a single dose
- once a dose is drawn up, it should be used within the manufacturer specified time or discarded at the end of the workday
- once a manufacturer-filled syringe is activated (i.e., needle attached or needle covered removed) it should be used or discarded at the end of the workday
Prepare vaccine just prior to administration. Agitate the vial to mix the vaccine thoroughly and obtain a uniform suspension prior to withdrawing each dose. Whenever solution and container permit, inspect the vaccine visually for discoloration, precipitation or if it cannot be re-suspended prior to administration. If problems are noted (e.g., vaccine cannot be re-suspended), the vaccine should not be administered.
Standard medication preparation guidelines should be followed for drawing a dose of vaccine into a syringe. A vaccine dose should not be drawn into the syringe until it is to be administered. The cap on top of a vaccine vial functions as a dust cover. Once removed, cleansing the exposed rubber stopper with a pre-packaged sterile alcohol wipe is recommended. Do not enter a vial with a used syringe or needle. Once the syringe(s) are filled, label the syringe with the type of vaccine. Administer the doses as soon as possible after filling. CDC recommends that providers draw up vaccines only at the time of administration. Do NOT predraw doses before they are needed. (See Vaccine Preparation in the Storage and Handling chapter) Medications packaged as single-use vials or syringes should never be used for more than one patient. Single-dose vials and manufacturer-filled syringes are designed for single-dose administration and should be discarded if vaccine has been withdrawn or reconstituted and subsequently not used within the time frame specified by the manufacturer.
Vaccines should never be combined in a single syringe except when specifically approved by the FDA and packaged for that specific purpose. Most combination vaccines will be combined by the manufacturer. As of March 2013, there are two binary vaccines (i.e. vaccines whose antigens are divided between freeze-dried portion and diluent) that must be combined by the provider at the time of administration, i.e., DTaP-IPV/Hib (Pentacel), and MCV4 (Menveo).
Vaccine should never be transferred from one syringe to another. Partial doses from separate vials should not be combined to obtain a full dose. Both of these practices increase the risk of contamination. Instilling air into a multidose vial prior to withdrawing a vaccine dose may not be necessary. It could cause a “spritz” of vaccine to be lost the next time the vial is entered, which through time can decrease the amount of vaccine in the vial and lead to the loss of a dose (e.g., obtaining only 9 full doses from a 10-dose vial).
Route and Site
Vaccine Preparation “Nevers”
- Never combine vaccines into a single syringe except when specifically approved by the FDA and packaged for that specific purpose
- Never transfer vaccine from one syringe to another
- Never draw partial doses of vaccine from separate vials to obtain a full dose
Oral (PO) Route Rotavirus Vaccines
- Administer oral vaccines, in general, prior to administering injections or performing other procedures that might cause discomfort
- Administer liquid slowly down one side of the inside cheek (between the cheek and gum) toward the back of infant’s mouth
- Take care not to go far enough back to initiate the gag reflex
- Never administer or spray (squirt) vaccine directly into the throat
- Do not readminister a dose of rotavirus vaccine if the infant regurgitates, spits out or vomits during or after administration
Intranasal (NAS) Route Live Attenuated Influenza Vaccine (LAIV)
- Use the special sprayer provided
- Seat the patient with head tilted back with a provider hand supporting the back of the patient’s head
- Instruct the patient to breathe normally
- Insert the tip of the sprayer and spray half the dose in one nostril then remove the dose divider clip and administer the other half-dose in the other nostril
- Health-care personnel who are immunosuppressed and require protective isolation should not administer LAIV
Subcutaneous (subcut) Route
- Site
- thigh for infants younger than 12 months of age
- upper outer triceps of arm for children older than 12 months and adults (can be used for infants if necessary)
- Needle gauge and length
- 23- to 25-gauge needle, 5/8- inch
- Technique
- follow standard medication administration guidelines for site assessment/selection and site preparation
- pinch up tissue at site
- insert needle at 45° angle and inject
- withdraw needle and apply light pressure to injection site for several seconds with gauze pad
The recommended route and site for each vaccine are based on clinical trials, practical experience and theoretical considerations. This information is included in the manufacturer’s product information for each vaccine, see manufacturers’ package insert. There are five routes used to administer vaccines. Deviation from the recommended route may reduce vaccine efficacy or increase local adverse reactions.
Oral (PO) Route
Rotavirus vaccines (RV1 [Rotarix] RV5, [RotaTeq]) and oral typhoid (TY21a [Vivotif]) are the only U.S.-licensed vaccines that are administered by the oral route. RV1 (Rotarix) requires reconstitution prior to oral administration. Oral vaccines should generally be administered prior to administering injections or performing other procedures that might cause discomfort. Administer the liquid slowly down one side of the inside of the cheek (between the cheek and gum) toward the back of the infant’s mouth. Care should be taken not to go far enough back to initiate the gag reflex. Never administer or spray (squirt) the vaccine directly into the throat. Detailed information on oral delivery of these vaccines is included in each manufacturer’s product information.
ACIP does not recommend readministering a dose of rotavirus vaccine to an infant who regurgitates, spits out, or vomits during or after administration. No data exist on the benefits or risks associated with readministering a dose. The infant should receive the remaining recommended doses of rotavirus vaccine following the routine schedule. There are no restrictions on the infant’s consumption of breast milk or any other liquid before or after administration of either of these vaccines.
Intranasal (NAS) Route
The live attenuated influenza vaccine (LAIV, FluMist) is currently the only vaccine administered by the nasal route. The vaccine dose (0.2 mL) is inside a special sprayer device. A plastic clip on the plunger divides the dose into two equal parts. The patient should be seated in an upright position with head tilted back. Instruct the patient to breathe normally. The provider should gently place a hand behind the patient’s head. The tip of the nasal sprayer should be inserted slightly into the nostril. Half of the contents of the sprayer (0.1 mL) are sprayed into the nostril. The dose-divider clip is then removed and the procedure is repeated in the other nostril. Detailed information on the nasal administration of LAIV is included in the manufacturer’s product information. The dose does not need to be repeated if the patient coughs, sneezes, or expels the dose in any other way.
It is possible for the LAIV spray to cause low-level contamination of the environment with vaccine virus, but there have been no reports of vaccine virus transmission by this route. No instances of illness or attenuated vaccine virus infections have occured among inadvertently exposed health-care personnel or immunocompromised patients. Health-care personnel at increased risk for influenza complications, including those with underlying medical conditions, pregnant women, persons 50 years of age or older, or with immunosuppressive conditions, may safely administer LAIV. The only exception is personnel with immunosuppression severe enough to require a protective environment (e.g., for hematopoietic cell transplant). However, healthcare personnel with this level of immunosuppression are not likely to be administering any vaccines.
Subcutaneous (subcut) Route
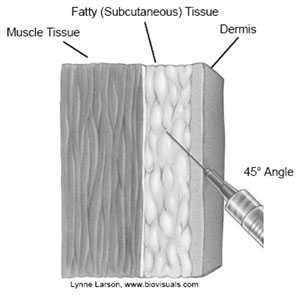
Source: California Department of Public Health
Source: California Department of Public Health
Subcutaneous Administration Technique
Source: California Department of Public Health
Subcutaneous injections are administered into the fatty tissue found below the dermis and above muscle tissue.
Site
The recommended subcutaneous sites for vaccine administration are the thigh (for infants younger than 12 months of age) and the upper outer triceps of the arm (for persons 1 year of age and older). If necessary, the upper outer triceps area can be used to administer subcutaneous injections to infants.
Needle Gauge and Length
5/8-inch, 23- to 25-gauge needle
Technique
- Follow standard medication administration guidelines for site assessment/selection and site preparation.
- To avoid reaching the muscle, pinch up the fatty tissue, insert the needle at a 45° angle and inject the vaccine into the tissue.
- Withdraw the needle and apply light pressure to the injection site for several seconds with a gauze pad.
Intramuscular (IM) Route
Intramuscular injections are administered into muscle tissue below the dermis and subcutaneous tissue.
Site
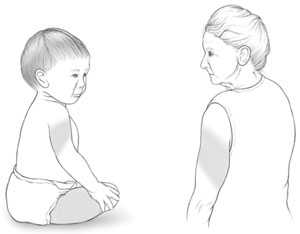
Intramuscular (IM) Route Infants 12 Months and Younger
- Site
- vastus lateralis muscle (anterolateral thigh)
- Needle gauge and length:
- 22- to 25-gauge
- neonates and preterm infants: 5/8-inch
- 5/8-inch needle is adequate only if the skin is stretched flat between thumb and forefinger
- 1 month and older: 1-inch
Intramuscular (IM) Route Toddlers 1 Year through 2 Years
- Site
- vastus lateralis muscle (anterolateral thigh) is preferred
- deltoid muscle (upper arm) may be used if the muscle mass is adequate
- Needle gauge and length
- 22- to 25-gauge
- 5/8 to 1-inch
- 5/8-inch needle is adequate only for the deltoid muscle and only if the skin is stretched flat between thumb and forefinger
Almost all inactivated vaccines are administered by the intramuscular route. Many inactivated vaccines contain an adjuvant, which is a vaccine component that enhances the immune response to the antigen. Adjuvants can cause an exaggerated local reaction (e.g., pain, swelling, redness) if not injected into the muscle, so proper technique is critical.
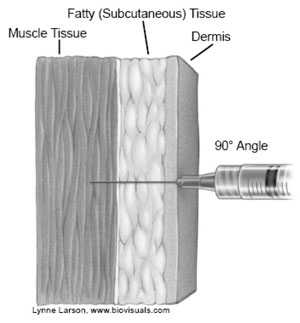
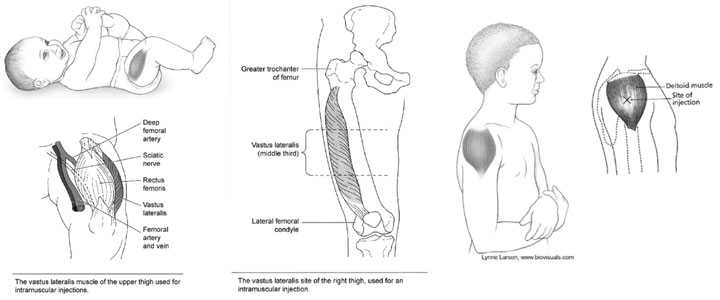
There are only two routinely recommended IM sites for administration of vaccines, the vastus lateralis muscle (anterolateral thigh) and the deltoid muscle (upper arm). Injection at these sites reduces the chance of involving neural or vascular structures. The preferred site depends on the age of the individual and the degree of muscle development.
Because there are no large blood vessels in the recommended sites, aspiration before injection of vaccines (i.e., pulling back on the syringe plunger after needle insertion but before injection) is not necessary. Also, some safety-engineered syringes do not allow for aspiration.
Two left images: Lynne Larson, www.biovisuals.com
Needle Gauge
22- to 25-gauge needle
Needle Length
The needle should be long enough to reach the muscle mass and prevent vaccine from seeping into subcutaneous tissue, but not so long as to involve underlying nerves, blood vessels, or bone. The health-care provider should be familiar with the anatomy of the area into which the vaccine will be injected.
Age-Based Recommendations
Decisions on needle size and site of injection must be made for each person on the basis of the size of the muscle, the thickness of adipose tissue at the injection site, the volume of the material to be administered, and injection technique.
Infants (12 Months and Younger)
Intramuscular (IM) Route Children/Adolescents 3 through 18 Years
- Site
- deltoid muscle (upper arm) is preferred
- vastus lateralis muscle (anterolateral thigh) may be used
- Needle gauge and length
- 22- to 25- gauge
- 5/8 to 1-inch
- 5/8-inch needle is adequate only for the deltoid muscle and only if the skin is stretched flat between thumb and forefinger
- Most young children in this age range require a 5/8 or 1-inch needle
- In general, older children and adolescents require a 1-inch needle
Intramuscular (IM) Route Adults 19 Years and Older
- Site:
- deltoid muscle (upper arm) is preferred
- vastus lateralis muscle (anterolateral thigh) may be used
- Needle gauge: 23- to 25-gauge
For the majority of infants, the anterolateral aspect of the thigh is the recommended site for injection because it provides a large muscle mass. The muscles of the buttock are not used for administration of vaccines in infants and children because of concern about potential injury to the sciatic nerve, which is well documented after injection of antimicrobial agents into the buttock. If the gluteal muscle must be used, care should be taken to define the anatomic landmarks. If the gluteal muscle is chosen, injection should be administered lateral and superior to a line between the posterior superior iliac spine and the greater trochanter or in the ventrogluteal site, the center of a triangle bounded by the anterior superior iliac spine, the tubercle of the iliac crest, and the upper border of the greater trochanter.
Injection technique is the most important parameter to ensure efficient intramuscular vaccine delivery. If the subcutaneous and muscle tissue are bunched to minimize the chance of striking bone, a 1-inch needle is required to ensure intramuscular administration in infants aged 1 month and older. For the majority of infants, a 1-inch, 22- to 25-gauge needle is sufficient to penetrate muscle in an infant’s thigh. For neonates (first 28 days of life) and preterm infants, a 5/8-inch needle usually is adequate if the skin is stretched flat between thumb and forefinger and the needle inserted at a 90-degree angle to the skin.
Toddlers (1 Year through 2 Years)
For toddlers, the vastus lateralis muscle in the anterolateral thigh is preferred. The needle should be at least 1-inch long. The deltoid muscle can be used if the muscle mass is adequate. A 5/8-inch needle is adequate only for the deltoid muscle and only if the skin is stretched flat between thumb and forefinger and the needle inserted at a 90° angle to the skin.
| Gender – Male | Gender – Female | Needle Length |
|---|---|---|
| Less than 130 pounds | Less than 130 pounds | 5/8 – 1-inch |
| 130 – 152 pounds | 130 – 152 pounds | 1-inch |
| 153 – 260 pounds | 153 – 200 pounds | 1 – 1.5-inches |
| 260+ pounds | 200+ pounds | 1.5-inches |
Children/Adolescents (3 through 18 Years)
The deltoid muscle is preferred for children aged 3 through 18 years of age. The needle size for deltoid injections can range from 22- to 25-gauge and from 5/8- to 1-inch, depending on technique. Most young children in this age range require a 5/8- or 1-inch needle. In general, older children and adolescents require a 1-inch needle. One study found that obese adolescents may need a 1½-inch needle in order to reach muscle tissue. If there is any doubt, knowledge of body mass may be helpful in estimating the appropriate needle length. The vastus lateralis muscle in the anterolateral thigh is an alternative site if the deltoid sites cannot be used. A 1- or 1¼-inch needle will be sufficient to reach muscle tissue in most older children and adolescents.
Adults (19 Years and Older)
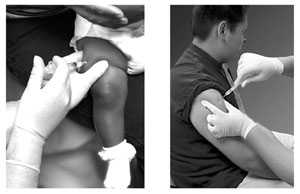
Intramuscular (IM) Injection Technique
- Follow standard medication administration guidelines for site assessment/selection and site preparation
- Spread the skin of the site taut between the thumb and forefinger, isolating the muscle
- Another technique, acceptable mostly for pediatric and geriatric patients, is to grasp the tissue and “bunch up” the muscle
- Insert the needle fully into the muscle at a 90° angle and inject
- Withdraw the needle and apply light pressure to the injection site for several seconds with a gauze pad
Intradermal (ID) Route
- Site
- deltoid region of the upper arm
- Needle gauge and length
- manufacturer prefilled microinjection syringe is used to administer a 0.1 mL dose into the dermal layer of the skin
- syringe contains a 30-gauge, 1.5 mL microneedle
- Technique
- hold the syringe between the thumb and the middle finger
- using a short quick motion insert the needle perpendicular to the skin
- push on the plunger with the index finger without aspirating
- after the vaccine is delivered, remove the syringe
- push firmly on the plunger until the needle shield is activated
For adults, the deltoid muscle is recommended for routine intramuscular vaccinations. The anterolateral thigh also can be used. For men and women weighing less than 130 lbs (60 kg) a 5/8 to 1-inch needle is sufficient to ensure intramuscular injection into the deltoid muscle if a 90° angle is used and the tissue is not bunched. For men and women who weigh 130-152 lbs (60-70 kg), a 1-inch needle is sufficient. For women who weigh 152-200 lbs (70-90 kg) and men who weigh 152-260 lbs (70-118 kg), a 1 to 1½-inch needle is recommended. For women who weigh more than 200 lbs (more than 90 kg) or men who weigh more than 260 lbs (more than 118 kg), a 1½-inch needle is recommended. As with adolescents, the vastus lateralis muscle in the anterolateral thigh is an alternative site if the deltoid sites cannot be used.
Technique
- Follow standard medication administration guidelines for site assessment/selection and site preparation.
- To avoid injection into subcutaneous tissue, spread the skin of the selected vaccine administration site taut between the thumb and forefinger, isolating the muscle. Another technique, acceptable mostly for pediatric and geriatric patients, is to grasp the tissue and “bunch up” the muscle.
- Insert the needle fully into the muscle at a 90° angle and inject the vaccine into the tissue.
- Withdraw the needle and apply light pressure to the injection site for several seconds with a gauze pad.
Intramuscular Administration Technique
Source: California Department of Public Health
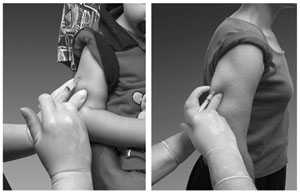
Intradermal (ID) Route
Fluzone Intradermal is the only U.S.-licensed vaccine that is administered by the intradermal route. This Fluzone formulation is not the same as intramuscular formulations of inactivated influenza vaccine (IIV). Other IIV formulations should NOT be administered by the intradermal route.
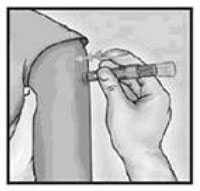
Site
The site of administration is the deltoid region of the upper arm. The patient should be seated with the arm bent at the elbow and the hand on the hip to ensure that the site of administration is prominent.
Source: Sanofi Pasteur Inc. 
Source: Sanofi Pasteur Inc. 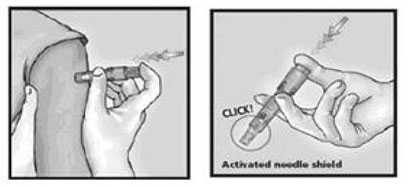
Source: Sanofi Pasteur Inc.
Needle Gauge and Length
A manufacturer prefilled microinjection syringe is used to administer a 0.1 mL dose into the dermal layer of the skin. The syringe contains a 30-gauge, 1.5 mL microneedle.
Technique
The syringe should be gently shaken before the needle cap is removed. Hold the syringe between the thumb and the middle finger. Using a short quick motion insert the needle perpendicular to the skin into the deltoid region of the upper arm. Push on the plunger with the index finger without aspirating. Because the needle is very short the vaccine will be delivered just under the skin into the dermal layer. This vaccine should NOT be administered into the volar aspect of the forearm or by the intradermal technique used to administer a tuberculin skin test.
After the vaccine is delivered, remove the syringe and point it away from anyone. Push firmly on the plunger with the thumb until a click is heard. A protective shield will cover the needle and the syringe can be disposed of in a sharps container.
Multiple Vaccinations
Multiple Vaccinations
- Administer each vaccine at a different anatomic site
- Use anterolateral thigh for infants and young children
- Use deltoid for older children and adults if muscle mass is adequate
- Separate injections by at least 1 inch, or more if possible
- Use a separate limb for most reactive vaccines (e.g., tetanus toxoid-containing and PCV13), if possible
- Use combination vaccines when appropriate to reduce the number of injections
If multiple vaccines are administered at a single visit, administration of each preparation at a different anatomic site is desirable. For infants and younger children, if more than two vaccines are injected in a single limb, the thigh is the preferred site because of the greater muscle mass. For older children and adults, the deltoid muscle can be used for more than one intramuscular injection. The injection sites should be separated by 1 inch or more, if possible, so that any local reactions can be differentiated. Vaccines that are the most reactive (e.g., tetanus toxoid-containing and PCV13) should be administered in different limbs if possible. Use of combination vaccines can reduce the number of injections. A number of job aids are available for immunization providers. See Giving All the Doses Under 12 Months [1 page], Giving All the Doses 12 Months and Older [1 page], and Giving All the Doses for Age 11 Years and Older [1 page].
If a vaccine and an immune globulin preparation are administered simultaneously (e.g., Td/Tdap and tetanus immune globulin [TIG] or hepatitis B vaccine and hepatitis B immune globulin [HBIG]), separate anatomic sites should be used.
The location of all injection sites should be documented in the patient’s medical record. Health-care providers should consider using a vaccination site map so that all persons administering vaccines routinely use the same anatomic site for each different vaccine.
Vaccinating Persons with Bleeding Disorders
- Individuals with bleeding disorder or receiving anticoagulant therapy may develop hematomas in IM injection sites
- Administer vaccines by recommended IM route IF physician familiar with patient’s bleeding risk determines vaccine can be safely administered
- Prior to vaccination, instruct about risk of hematoma
- Schedule shortly after antihemophelia or similar therapy
- Use 23-gauge or finer needle and apply firm pressure to injection site for at least 2 minutes after injection
- Do NOT rub or massage injection site
Vaccinating Persons with Bleeding Disorders
Individuals with a bleeding disorder or who are receiving anticoagulant therapy may develop hematomas in IM injection sites. When any intramuscularly administered vaccine is indicated for a patient with a bleeding disorder, the vaccine should be administered intramuscularly if a physician familiar with the patient’s bleeding risk determines that the vaccine can be administered by this route with reasonable safety. Prior to administration of IM vaccines the patient or family should be instructed about the risk of hematoma formation from the injection. If the patient periodically receives antihemophilia or similar therapy, IM vaccine administration should be scheduled shortly after such therapy is administered. A 23-gauge or finer needle should be used and firm pressure applied to the site for at least 2 minutes after injection. The site should not be rubbed or massaged. Patients receiving anticoagulation therapy presumably have the same bleeding risk as patients with clotting factor disorders and providers should follow the same guidelines for intramuscular administration.
Nonstandard Administration
- CDC discourages deviation from recommended route, site, dosage, or number of vaccine doses
- Revaccination is recommended if :
- hepatitis B vaccine is administered by any route other than IM or in any site of an adult other than deltoid or anterolateral thigh
- rabies vaccine is administered in gluteal site
- HPV vaccine is administered by any route other than IM
- less than the standard dose is administered unless serologic testing indicates an adequate response
- if a partial dose of a parenteral vaccine is administered because the syringe or needle leaks or the patient jerks away
Nonstandard Administration
CDC discourages deviating from the recommended route, site, dosage, or number of doses for any vaccine. Deviation can result in reduced protection and increase the risk of an exaggerated local reaction. For certain vaccines, the ACIP recommends revaccination if a nonstandard route or site is used. Hepatitis B vaccine administered by any route other than the intramuscular route, or in adults at any site other than the deltoid or anterolateral thigh, should not be counted as valid and should be repeated. Doses of rabies vaccine administered in the gluteal site should not be counted as valid doses and should be repeated. Revaccination is recommended when HPV vaccine is administered by any route other than IM. All vaccines should be administered by the manufacturer’s recommended route, but there are no ACIP recommendations to repeat doses of other vaccines administered by another route. For additional information, see the ACIP General Recommendations [64 pages].
Larger than recommended dosages can be hazardous because of excessive local or systemic concentrations of antigens or other vaccine constituents deposited into the tissue. Administering volumes smaller than recommended (e.g., inappropriately divided doses) might result in inadequate protection. Using reduced doses administered at multiple vaccination visits that equal a full dose or using smaller divided doses is not recommended. In addition, some vaccines (e.g., IIV, HepB, HepA) require different dosages (amount) based on the patient’s age. Any vaccination using less than the standard dose should not be counted, and the person should be revaccinated according to age unless serologic testing indicates that an adequate response has developed. If a partial dose of a parenteral vaccine is administered because the syringe or needle leaks or the patient jerks away, the dose should be repeated.
Managing Acute Vaccine Reactions
Severe, life-threatening anaphylactic reactions following vaccination are rare. Thorough screening for contraindications and precautions prior to vaccination can often prevent reactions. Staff must have in place and be familiar with procedures for managing a reaction. Staff should be familiar with the signs and symptoms of anaphylaxis because they usually begin within minutes of vaccination. These signs and symptoms can include, but are not limited to: flushing, facial edema, urticaria, itching, swelling of the mouth or throat, wheezing, and difficulty breathing. Each staff member should know their role in the event of an emergency and all vaccination providers should be certified in cardiopulmonary resuscitation (CPR). Epinephrine and equipment for maintaining an airway should be available for immediate use. After the patient is stabilized, arrangements should be made for immediate transfer to an emergency facility for additional evaluation and treatment, see Medical Management of Vaccine Reactions in Children and Teens [3 pages] and Medical Management of Vaccine Reactions in Adult Patients [2 pages].
Documentation
Documentation in Permanent Medical Record
- Required for vaccines covered by National Childhood Vaccine Injury Act
- date of administration
- vaccine manufacturer
- vaccine lot number
- name and title of person who administered vaccine and address of facility where permanent record will reside
- vaccine information statement (VIS)
- date on VIS
- date provided to patient or parent/guardian
- Best practice documentation
- vaccine type (ACIP abbreviation)
- route
- dosage (volume)
- site
- Document vaccine refusal
Immunization Information System (IIS)/ Registry
- Confidential, population-based, computerized database
- All providers are encouraged to use IIS/registry
- Some state IIS utilize barcoding technology
- 2D barcodes on some vaccine vials and VISs
All vaccines administered should be fully documented in the patient’s permanent medical record. Healthcare providers who administer vaccines covered by the National Childhood Vaccine Injury Act are required to ensure that the permanent medical record of the recipient indicates:
- Date of administration
- Vaccine manufacturer
- Vaccine lot number
- Name and title of the person who administered the vaccine and the address of the facility where the permanent record will reside
- Vaccine information statement (VIS)
- date printed on the VIS
- date VIS given to patient or parent/guardian
Best practice documentation guidelines for medications also include the vaccine type. See the ACIP U.S. Vaccine Abbreviations list, route, dosage (volume), and site. Accurate documentation can help prevent administration errors and curtail the number and costs of excess vaccine doses administered. Providers also should update patients’ permanent medical records to reflect any documented episodes of adverse events after vaccination and any serologic test results related to vaccine-preventable diseases (e.g., those for rubella screening and antibody to hepatitis B surface antigen). Participation in immunization information systems is encouraged. See additional documentation resources. The patient or parent/guardian should be provided with an immunization record that includes the vaccines administered, including the dates of administration.
Although there is no national law, it is also important to document when parents or adult patients refuse vaccine despite the immunization providers’ recommendation. Many professional organizations such as the American Academy of Pediatrics and others have developed forms to document when vaccines are refused. See Decision to Not Vaccinate My Child [2 pages] and Refusal to Consent to Vaccination (Adult) [1 page] for examples.
Immunization information systems (IIS) or registries are confidential, population-based, computerized databases in which immunization doses administered by participating providers to persons residing within a given geopolitical area can be documented. All immunization providers are encouraged to participate and document administered vaccines in an IIS. See additional information regarding Immunization Information Systems.
Some states’ IIS are able to utilize barcoding technology. Implementation of a 2D barcode on vaccine vials and VISs will allow for rapid, accurate, and automatic capture of certain data, including vaccine product identifier, lot number, and expiration date, and VIS edition date using a handheld imaging device, or scanner, which could populate these fields in an electronic health record (EHR) and/or an IIS. See additional information on barcoding and vaccines.
Strategies to Prevent Administration Errors
Strategies to Prevent Errors
- Adhere to “Rights of Medication Administration”
- Provide ongoing staff training and education
- Involve staff in selection of products to be used
- Use standardized ACIP vaccine abbreviations
- Keep current reference materials available for staff
- Rotate vaccines so those with shortest expiration dates are in front and check frequently to remove any expired vaccines
- Do not store sound-alike and look-alike vaccines next to each other
- Color code and label vaccines with type, age, and gender, if applicable
- Store pediatric and adult vaccines on separate shelves
- Administer only vaccines that you have prepared
- Triple check your work before administering a vaccine
- Avoid interruptions when selecting and preparing vaccines
- Consider using standing orders
- Counsel parents and patients about vaccines to be administered and about importance of maintaining personal immunization records
- Establish an environment that values reporting and investigating errors as part of risk management and quality improvement
- Promote a “just culture” where staff is willing to report errors trusting that the situation and those involved will be treated fairly
- Error reporting should provide opportunities to discover how errors occur and to share ideas to prevent or reduce those errors without fear of punishment and ridicule
Vaccine administration errors can result in a patient receiving an ineffective immunization. This can leave the person vulnerable to infection. Vaccine administration errors may also diminish patient confidence in their healthcare providers. Common vaccine administration errors include:
- Doses administered too early (before the minimum age or interval has been met)
- Wrong vaccine (e.g., Tdap instead of DTaP)
- Wrong dosage (e.g., pediatric formulation of hepatitis B vaccine administered to an adult)
- Wrong route
- Vaccine administered outside the approved age range
- Expired vaccine or diluent administered
- Vaccine which was not stored properly administered
- Vaccine administered to a patient with a contraindication for that vaccine
- Wrong diluent used to reconstitute the vaccine or only the diluent was administered
In addition to strict adherence to the “Rights of Medication Administration” and ongoing training and education of staff, there are other strategies that can be implemented to help prevent administration errors.
When possible, involve staff in the selection of vaccine products to be used in your facility. Different brands of the same vaccine can have different schedules, age indications, or other indications. Stocking multiple brands may lead to staff confusion and vaccine administration errors.
Use standardized abbreviations to avoid confusion about which vaccines have been administered. See ACIP Abbreviations for Vaccines.
Keep current reference materials available for staff on each vaccine used in your facility. Keep reference sheets for timing and spacing, recommended sites, routes, and needle lengths posted for easy reference in your medication preparation area. For additional information, see clinic resources for administering vaccines.
Rotate vaccines so that those with the shortest expiration dates are in the front of the storage unit. Use these first and frequently check the storage unit to remove any expired vaccine.
Consider the potential for product mix-ups when storing vaccines. Do not store sound-alike and look-alike vaccines next to each other (e.g., DTaP and Tdap). Consider color coding labels on vaccine storage containers and/or including the vaccine type, age indications, and gender if applicable. Store the pediatric and adult vaccines on separate shelves in the storage unit. See Vaccine Label Examples [18 pages].
Administer only vaccines that you have prepared for administration. Triple check your work before you administer a vaccine and ask other staff to do the same.
Avoid interruptions when selecting and preparing the appropriate vaccine(s) for administration.
Consider using standing orders if appropriate for your facility. Standing orders provide protocols for administering vaccines in a consistent, systematic format. For standing order templates, see Standing Orders for Administering Vaccines.
Counsel parents and patients about vaccines to be administered and on how important it is for them to maintain immunization records on all family members. Educated clients may notice a potential error and help prevent it.
Establish an environment that values the reporting and investigation of errors as part of risk management and quality improvement. Promote a “just culture” where staff is willing to report errors trusting that the situation and those involved will be treated fairly. Error reporting should provide opportunities to discover how the errors occur and to share ideas to prevent or reduce those errors in the future without fear of punishment and ridicule.
VAERS
- VAERS accepts all reports, including reports of vaccination errors
- VAERS is primarily concerned with monitoring adverse health events and encourages
reporting of clinically significant adverse health events following vaccination - Healthcare professionals can decide whether or not to report a medical error at their own discretion
- if they think the vaccination error may pose a safety risk (e.g., administering a live vaccine to an immunocompromised patient)
- the error would be preventable with public health action or education
Vaccine Adverse Event Reporting System (VAERS)
The Vaccine Adverse Event Reporting System (VAERS) accepts all reports of adverse events occurring with vaccinations, including reports of vaccination errors. VAERS is primarily concerned with monitoring adverse health events and encourages reporting of clinically significant adverse health events following vaccination. Using clinical judgment, healthcare professionals can decide whether or not to report a medical error at their own discretion. For example, a healthcare professional may elect to report vaccination errors that do not have an associated adverse health event, especially if they think the vaccination error may pose a safety risk (e.g., administering a live vaccine to an immunocompromised patient) or that the error would be preventable with public health action or education.
Acknowledgement
The editors thank JoEllen Wolicki, Dr. Cindy Weinbaum, and Donna Weaver, CDC, for their contribution to this chapter.
Selected References and Resources
- Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices [64 pages]. MMWR 2011;60(no. RR-2): 23-27.
- Centers for Disease Control and Prevention (CDC). Injection Practices: Information for Providers
- Centers for Disease Control and Prevention (CDC). Recommendations and Guidelines: Vaccine Administration
- Cohen, Michael R. Medication Errors, 2nd ed. Washington, D.C.: American Pharmacists Assoc.; 2007.
- Harrington JW, Logan SJ, Harwell C, et al. Effective analgesia using physical interventions for infant immunizations. Pediatrics 2012;129: 815-21.
- Immunization Action Coalition (IAC). Various resources on vaccine administration
- Ipp M, Taddio A, Sam J, et al. Vaccine-related pain: randomized controlled trial of two injection techniques. Arch Dis Child 2007;92: 1105-08.
- Reis EC, Roth EK, Syphan JL, et al. Effective pain reduction for multiple immunization injections in young infants. Arch Pediatr Adolesc Med 2003;157: 115-1120.
- Smith, SF, Duell DJ, and Martin, BC. Clinical Nursing Skills: Basic to Advanced Skills, 8th ed. Saddle River, NJ: Pearson Education, Inc.; 2012.
- Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain childhood vaccination: an evidence-based clinical practice guideline. Can Med Assc Journal 2010;182:E843-E855.
- Taddio A, Ilersich AL, Ipp M, et al. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations systematic review of randomized controlled trials and quasi-randomized controlled trials. Clinical Therapeutics Supplement B 2009;31: S48-S76.
- Taddio A, Chambers C, Halperin S, et al. Inadequate pain management during routine childhood immunizations: the nerve of it. Clinical Therapeutics Supplement B 2009;31: S152-S167.
- Page last reviewed: November 15, 2016
- Page last updated: September 8, 2015
- Content source:


 ShareCompartir
ShareCompartir