Influenza Antiviral Drug Resistance
Questions & Answers
On This Page
- What is antiviral resistance?
- How does antiviral resistance happen?
- How is antiviral resistance detected?
- What is oseltamivir resistance?
- What causes oseltamivir resistance?
- How does CDC improve monitoring of influenza viruses for antiviral resistance?
- How did influenza antiviral resistance patterns change during the previous (2015-2016) influenza season?
- What antiviral drugs are recommended for use during the 2016-2017 flu season?
- What can people do to protect themselves against antiviral resistant flu viruses?
- What implications does antiviral resistance have for the U.S. antiviral stockpile that was created as part of the United States pandemic plans?
What is antiviral resistance?
Antiviral resistance means that a virus has changed in such a way that antiviral drugs are less effective or not effective at all in treating or preventing illnesses with that virus. Influenza viruses can become resistant to influenza antiviral drugs. In the United States, there are three FDA-approved neuraminidase inhibitor antiviral drugs recommended by CDC this season: oseltamivir (available as a generic version or under the trade name Tamiflu®), zanamivir (trade name Relenza®), and peramivir (trade name Rapivab®). In the United States, most of the recently circulating influenza viruses have been susceptible to the neuraminidase inhibitor antiviral medications.
There is another class of influenza antiviral drugs (amantadine and rimantadine) called the adamantanes that are not recommended for use in the United States at this time. Many flu A viruses are resistant to these drugs and they are not effective against flu B viruses.
How does antiviral resistance happen?
Influenza viruses are constantly changing; they can change from one season to the next and can even change within the course of one flu season. As a flu virus replicates (i.e., make copies of itself), the genetic makeup may change in a way that results in the virus becoming resistant to one or more of the antiviral drugs used to treat or prevent influenza. Influenza viruses can become resistant to antiviral drugs spontaneously or emerge during the course of antiviral treatment. Drug-resistant viruses vary in their ability to transmit to other people.
How is antiviral resistance detected?
CDC routinely collects flu viruses through a domestic and global surveillance and tests them to see if they are resistant to any of the FDA-approved flu antiviral drugs. This data informs public health policy recommendations about the use of flu antiviral medications. Antiviral resistance testing involves several laboratory tests, including a specific functional assay, the neuraminidase inhibition (NI) assay, and molecular techniques (sequencing and pyrosequencing) to look for genetic changes that are associated with antiviral resistance.
What is oseltamivir resistance?
Oseltamivir is an antiviral drug that is used to treat flu illness. “Oseltamivir resistance” refers to a flu virus that is resistant to the drug oseltamivir. Similarly, influenza viruses can become resistant to other influenza antiviral drugs.
What causes oseltamivir resistance?
Flu viruses are constantly changing (for more information, see How the Flu Virus Can Change). Changes that occur in circulating flu viruses typically involve the structures of the viruses’ two primary surface proteins: neuraminidase (NA) and hemagglutinin (HA). (See image below for a visualization of a flu virus and its HA and NA surface proteins.)
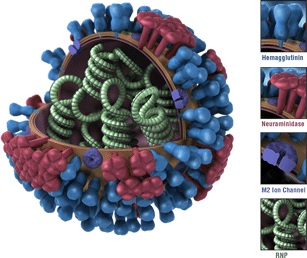
Oseltamivir is known as a “NA inhibitor” because this antiviral drug binds to a flu virus’ NA and inhibits the enzymatic activity of this protein. By inhibiting NA activity, oseltamivir prevents flu viruses from spreading from infected cells to other healthy cells. However, if the NA proteins of flu virus change, oseltamivir can lose its ability to bind to and inhibit the function of the virus’s NA proteins. This results in oseltamivir resistance (non-susceptibility). A particular genetic change known as the “H275Y” mutation is known to confer oseltamivir resistance in 2009 H1N1 flu viruses. (The H275Y mutation is a substitution of histidine for tyrosine at position 275 in the NA.) This substitution prevents oseltamivir from inhibiting NA activity and allows the mutated virus to spread to healthy cells, which results in the drug not working as well.
How does CDC improve monitoring of influenza viruses for antiviral resistance?
CDC continually improves the ability to rapidly detect and monitor antiviral resistance through improvements in laboratory methods and by increasing the number of surveillance sites domestically and globally and increasing the number of laboratories that can test for antiviral resistance. Enhanced surveillance efforts have provided CDC with the capability to detect resistant viruses more quickly, and enabled CDC to monitor for changing trends over time.
How did influenza antiviral resistance patterns change during the previous (2015-2016) influenza season?
Antiviral resistance patterns did not change in 2015-16 compared with the previous season. In both seasons, oseltamivir resistance was found in a small number of H1N1 viruses. Most of the influenza viruses tested during 2015-2016 continued to be susceptible to the antiviral drugs recommended for influenza by the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) (oseltamivir, zanamivir and peramivir) while resistance to the adamantanes class of antiviral drugs among A/H3N2 and A/H1N1 viruses remains widespread (influenza B viruses are not susceptible to adamantine drugs).
Specifically, for the 2015-2016 season:
- 99.2% of the tested H1N1pdm09 viruses were susceptible to oseltamivir and peramivir, and 100% of the H1N1pdm09 viruses tested were susceptible to zanamivir.
- 100% of influenza A (H3N2) tested were susceptible to oseltamivir, zanamivir, and peramivir; and;
- 100% of influenza B viruses tested were susceptible to oseltamivir, zanamivir, and peramivir.
- High levels of resistance to the adamantanes (amantadine and rimantadine) persist among the influenza A viruses currently circulating. The adamantanes are not effective against influenza B viruses. Adamantane drugs are not recommended for use against influenza at this time
Because there were no dramatic changes in antiviral resistance patterns during 2015-2016 flu season, the 2016-2017 guidance on the use of influenza antiviral drugs remains unchanged.
CDC conducts ongoing surveillance and testing of influenza viruses for antiviral resistance among seasonal and novel influenza viruses, and guidance is updated as needed.
What antiviral drugs are recommended for use during the 2016-2017 flu season?
Antiviral medications currently recommended include oseltamivir, zanamivir, and peramivir. The vast majority of currently circulating influenza viruses are sensitive to these medications. Rare exceptions have been detected. Note: currently circulating flu viruses have high levels of resistance to the adamantane class of antiviral drugs (which includes amantadine and rimantadine), and therefore, these drugs are not recommended for use in the United States at this time.
What can people do to protect themselves against antiviral resistant flu viruses?
Getting a yearly seasonal flu vaccination is the first and most important step in preventing the flu. The vaccine protects against an influenza A (H1N1) virus, an influenza A (H3N2) virus, and one or two influenza B viruses (depending on the vaccine). CDC recommends that everyone 6 months of age and older get vaccinated each year. If you are in a group at high risk of serious flu-related complications and become ill with flu symptoms, call your doctor right away, you may benefit from early treatment. If you are not at high risk, if possible, stay home from work, school and errands when you are sick. This will help prevent you from spreading your illness to others. See Important Information For People Sick With Flu for more information.
What implications does antiviral resistance have for the U.S. antiviral stockpile that was created as part of the United States pandemic plan?
Antiviral drugs are one component of a multifaceted approach to pandemic preparedness planning and response. The U.S. influenza antiviral drug stockpile includes supplies of both of the NA inhibitor agents, oseltamivir and zanamivir. These medications are to be used in the event that a novel influenza A subtype virus, such as the avian influenza A (H5N1) virus, emerges and begins to spread easily among humans. During the 2009 H1N1 pandemic, antiviral drugs were released from the Strategic National Stockpile (SNS) and used to treat infection with the 2009 influenza A (H1N1) virus. Information about how antiviral drugs from the stockpile were used during the 2009 H1N1 pandemic is available in The 2009 H1N1 Pandemic: Summary Highlights, April 2009-April 2010. The antiviral stockpile is for public health emergencies in the United States, such as an influenza pandemic, not to provide medication for the treatment of seasonal influenza.
- Page last reviewed: January 20, 2017
- Page last updated: January 26, 2017
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir