Overview of Influenza Surveillance in the United States
The Epidemiology and Prevention Branch in the Influenza Division at CDC collects, compiles and analyzes information on influenza activity year-round in the United States and produces FluView, a weekly influenza surveillance report, and FluView Interactive, which allows for more in-depth exploration of influenza surveillance data. The U.S. influenza surveillance system is a collaborative effort between CDC and its many partners in state, local, and territorial health departments, public health and clinical laboratories, vital statistics offices, healthcare providers, clinics, and emergency departments. Information in five categories is collected from eight different data sources that allows CDC to:
- Find out when and where influenza activity is occurring
- Track influenza-related illness
- Determine what influenza viruses are circulating
- Detect changes in influenza viruses
- Measure the impact influenza is having on hospitalizations and deaths
Five Categories of Influenza Surveillance
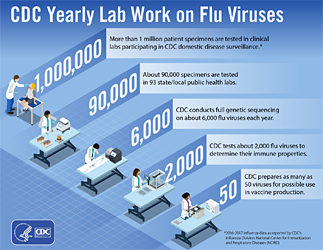
1. Virologic Surveillance — Approximately 100 public health and over 300 clinical laboratories located throughout all 50 states, Puerto Rico, and the District of Columbia participate in virologic surveillance for influenza through either the U.S. World Health Organization (WHO) Collaborating Laboratories System or the National Respiratory and Enteric Virus Surveillance System (NREVSS). Influenza testing practices differ in public health and clinical laboratories and both sources provide valuable information for monitoring influenza activity. Clinical laboratories primarily test respiratory specimens for diagnostic purposes and data from these laboratories provide useful information on the timing and intensity of influenza activity. Public health laboratories primarily test specimens for surveillance purposes to understand what influenza viruses are circulating throughout their jurisdiction and the population groups being affected. A subset of specimens from clinical laboratories may be submitted to public health laboratories for further testing. In order to use each data source most appropriately and to avoid duplication, reports from public health and clinical laboratories have been presented separately in both FluView and FluView Interactive beginning in the 2015-2016 influenza season. All public health and clinical laboratories report each week to CDC the total number of respiratory specimens tested and the number positive for influenza viruses, along with age or age group of the person, if available. Data presented from clinical laboratories include the weekly total number of specimens tested, the number of positive influenza tests, and the percent positive by influenza virus type. Data presented from public health laboratories include the weekly total number of specimens tested, the number of positive influenza tests, and the number by influenza virus type, subtype, and influenza B lineage. In order to obtain enough specimens to produce this detailed information in an efficient manner, public health laboratories often receive samples that have already tested positive for an influenza virus at a clinical laboratory. As a result, monitoring the percent of specimens testing positive for an influenza virus in a public health laboratory is less useful (i.e., we expect a higher percent positive). Fortunately, it is not necessary to monitor this parameter when clinical laboratory data are available instead.
In addition, the age distribution of influenza positive specimens reported from public health laboratories is visualized in FluView and FluView Interactive. The number and proportion of influenza virus-positive specimens by influenza A subtype and influenza B virus lineage are presented by age group (0-4 years, 5-24 years, 25-64 years, and ≥65 years) each week and cumulative totals are provided for the season. A subset of the influenza viruses collected by public health laboratories are sent to CDC for further characterization, including antiviral resistance testing and antigenic and/or genetic characterization, and this information is presented in the antiviral resistance and virus characterization sections of the FluView report.
Surveillance for Novel Influenza A Viruses – In 2007, human infection with a novel influenza A virus became a nationally notifiable condition. Novel influenza A virus infections include all human infections with influenza A viruses that are different from currently circulating human seasonal influenza H1 and H3 viruses. These viruses include those that are subtyped as nonhuman in origin and those that are not able to be subtyped with standard laboratory methods and reagents. Rapid detection and reporting of human infections with novel influenza A viruses – viruses against which there is little to no pre-existing immunity – is important to facilitate prompt awareness and characterization of influenza A viruses with pandemic potential and accelerate the implementation of effective public health responses.
Top of Page2. Outpatient Illness Surveillance — Information on patient visits to health care providers for influenza-like illness is collected through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet). ILINet consists of more than 2,800 enrolled outpatient healthcare providers in all 50 states, Puerto Rico, the District of Columbia and the U.S. Virgin Islands reporting more than 39 million patient visits each year. Each week, approximately 2,000 outpatient healthcare providers around the country report data to CDC on the total number of patients seen for any reason and the number of those patients with influenza-like illness (ILI) by age group (0-4 years, 5-24 years, 25-49 years, 50-64 years, and ≥65 years). For this system, ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza. Sites with electronic health records use an equivalent definition as determined by public health authorities.
- The percentage of patient visits to healthcare providers for ILI reported each week is weighted on the basis of state population. This percentage is compared each week with the national baseline of 2.2%. The baseline is developed by calculating the mean percentage of patient visits for ILI during non-influenza weeks for the previous three seasons and adding two standard deviations. A non-influenza week is defined as periods of two or more consecutive weeks in which each week accounted for less than 2% of the season’s total number of specimens that tested positive for influenza in public health laboratories. Due to wide variability in regional level data, it is not appropriate to apply the national baseline to regional data; therefore, region specific baselines are calculated using the same methodology.
Regional baselines for the 2017-2018 influenza season are:
Region 1 — 1.4%
Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont
Region 2 — 3.1%
New Jersey, New York, Puerto Rico, and the U.S. Virgin Islands
Region 3 — 2.0%
Delaware, District of Columbia, Maryland, Pennsylvania, Virginia, and West Virginia
Region 4 — 1.9%
Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee
Region 5 — 1.8%
Illinois, Indiana, Michigan, Minnesota, Ohio, and Wisconsin
Region 6 — 4.2%
Arkansas, Louisiana, New Mexico, Oklahoma, and Texas
Region 7 — 1.9%
Iowa, Kansas, Missouri, and Nebraska
Region 8 — 1.3%
Colorado, Montana, North Dakota, South Dakota, Utah, and Wyoming
Region 9 — 2.4%
Arizona, California, Hawaii, and Nevada
Region 10— 1.4%
Alaska, Idaho, Oregon, and Washington
- ILI Activity Indicator Map: — Data collected in ILINet are used to produce a measure of ILI activity for all 50 states, Puerto Rico, the District of Columbia, and New York City. Activity levels are based on the percent of outpatient visits in a jurisdiction due to ILI compared with the average percent of ILI visits that occur during weeks with little or no influenza virus circulation (i.e., non-influenza weeks). Because the number of sites reporting each week is variable, baselines are adjusted each week based on which sites within each jurisdiction provide data. To perform this adjustment, provider level baseline ratios are calculated for those that have a sufficient reporting history. Providers that do not have the required reporting history are assigned the baseline ratio for their practice type. The jurisdiction level baseline is then calculated using a weighted sum of the baseline ratios for each contributing provider.The activity levels compare the mean reported percent of visits due to ILI for the current week to the mean reported percent of visits due to ILI for non-influenza weeks. The 10 activity levels correspond to the number of standard deviations below, at or above the mean for the current week compared with the mean of the non-influenza weeks. There are 10 activity levels classified as minimal (levels 1-3), low (levels 4-5), moderate (levels 6-7), and high (levels 8-10). An activity level of 1 corresponds to values that are below the mean, level 2 corresponds to an ILI percentage less than 1 standard deviation above the mean, level 3 corresponds to ILI more than 1, but less than 2 standard deviations above the mean, and so on, with an activity level of 10 corresponding to ILI 8 or more standard deviations above the mean.
3. Mortality Surveillance — Rapid tracking of influenza-associated deaths is done through two systems:
- National Center for Health Statistics (NCHS) mortality surveillance data – NCHS collects death certificate data from state vital statistics offices for all deaths occurring in the United States. Pneumonia and influenza (P&I) deaths are identified based on ICD-10 multiple cause of death codes. NCHS surveillance data are aggregated by the week of death occurrence and as a result, P&I percentages based on the NCHS surveillance data are released two weeks after the week of death to allow for collection of enough data to produce a stable P&I percentage. The NCHS surveillance data based on P&I percentage for earlier weeks are continually revised and may increase or decrease as new and updated death certificate data are received from the states by NCHS. The seasonal baseline of P&I deaths is calculated using a periodic regression model that incorporates a robust regression procedure applied to data from the previous five years. An increase of 1.645 standard deviations above the seasonal baseline of P&I deaths is considered the “epidemic threshold,” i.e., the point at which the observed proportion of deaths attributed to pneumonia or influenza was significantly higher than would be expected at that time of the year in the absence of substantial influenza-related mortality.
- Influenza-Associated Pediatric Mortality Surveillance System — Influenza-associated deaths in children (persons less than 18 years) was added as a nationally notifiable condition in 2004. Any laboratory-confirmed influenza-associated death in a child is reported through this system. Demographic and clinical information are collected on each case and are transmitted to CDC.
4. Hospitalization Surveillance — Laboratory confirmed influenza-associated hospitalizations in children and adults are monitored through the Influenza Hospitalization Surveillance Network (FluSurv-NET).
- Influenza Hospitalization Surveillance Network (FluSurv-NET) — FluSurv-NET conducts surveillance for population-based, laboratory-confirmed influenza related hospitalizations in children (persons less than 18 years) and adults. The network covers over 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and three additional states (MI, OH, and UT). Cases are identified by reviewing hospital laboratory and admission databases and infection control logs for patients hospitalized during the influenza season with a documented positive influenza test (i.e., viral culture, direct/indirect fluorescent antibody assay (DFA/IFA), rapid influenza diagnostic test (RIDT), or molecular assays including reverse transcription-polymerase chain reaction (RT-PCR)). Data gathered are used to estimate age-specific hospitalization rates on a weekly basis during the influenza season and to describe characteristics of persons hospitalized with associated influenza illness. Laboratory confirmation is dependent on clinician-ordered influenza testing. Therefore, the rates provided are likely to be underestimated as influenza-associated hospitalization can be missed, either because testing was not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
5. Summary of the Geographic Spread of Influenza — State health departments report the estimated level of geographic spread of influenza activity in their states each week through the State and Territorial Epidemiologists Reports. States report geographic spread of influenza activity as no activity, sporadic, local, regional, or widespread. These levels are defined as follows:
- No Activity: No laboratory-confirmed cases of influenza and no reported increase in the number of cases of ILI.
- Sporadic: Small numbers of laboratory-confirmed influenza cases or a single laboratory-confirmed influenza outbreak has been reported, but there is no increase in cases of ILI.
- Local: Outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in a single region of the state.
- Regional: Outbreaks of influenza or increases in ILI and recent laboratory confirmed influenza in at least two but less than half the regions of the state with recent laboratory evidence of influenza in those regions.
- Widespread: Outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least half the regions of the state with recent laboratory evidence of influenza in the state.
Together, the five categories of influenza surveillance are designed to provide a national picture of influenza activity. FluSurv-NET data provide population-based, laboratory-confirmed estimates of influenza-related hospitalizations but are reported from limited geographic areas. NCHS mortality surveillance data are reported on the national, Department of Health and Human Services (HHS) regional, and state-level. Outpatient influenza-like illness and laboratory data are reported on a national level, by HHS region, and by states who have elected to release their data on the state-level. The state and territorial epidemiologists’ reports of the geographic spread of influenza activity and the ILI activity indicator map display state-level information. Human infections with novel influenza A viruses and influenza-associated pediatric deaths are reported on the state-level but no personal identifying information is released.
It is important to maintain a comprehensive system for influenza surveillance for several reasons:
- Influenza viruses are constantly changing and thus ongoing data collection and characterization of the viruses are required.
- Influenza viruses can rapidly undergo changes leading to pandemics of influenza; surveillance of viruses will detect these changes.
- Vaccines must be administered annually and are updated regularly based on surveillance findings.
- Treatment for influenza is guided by laboratory surveillance for antiviral resistance.
- National responses to emerging pandemic strains are triggered by surveillance data.
- Varying segments of the population are affected by influenza and may require targeted interventions. These groups are determined through influenza surveillance.
It is important to remember the following about influenza surveillance in the United States:
- All influenza activity reporting by public health partners and health-care providers is voluntary.
- The reported information answers the questions of where, when, and what influenza viruses are circulating. It can be used to determine if influenza activity is increasing or decreasing, but cannot be used to ascertain how many people have become ill with influenza during the influenza season.
- The system consists of eight complementary surveillance components in five categories. These components include reports from more than 350 laboratories, 2,800 outpatient health care providers, the National Center for Health Statistics, research and healthcare personnel at the FluSurv-NET sites, and influenza surveillance coordinators and state epidemiologists from all state, local and territorial health departments.
- Influenza surveillance data collection are based on a reporting week that starts on Sunday and ends on Saturday of each week. Each surveillance participant is requested to summarize weekly data and submit it to CDC by Tuesday afternoon of the following week. Those data are then downloaded, compiled, and analyzed at CDC. FluView and FluView Interactive will be updated weekly each Friday.
- Page last reviewed: October 13, 2017
- Page last updated: October 13, 2017
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir