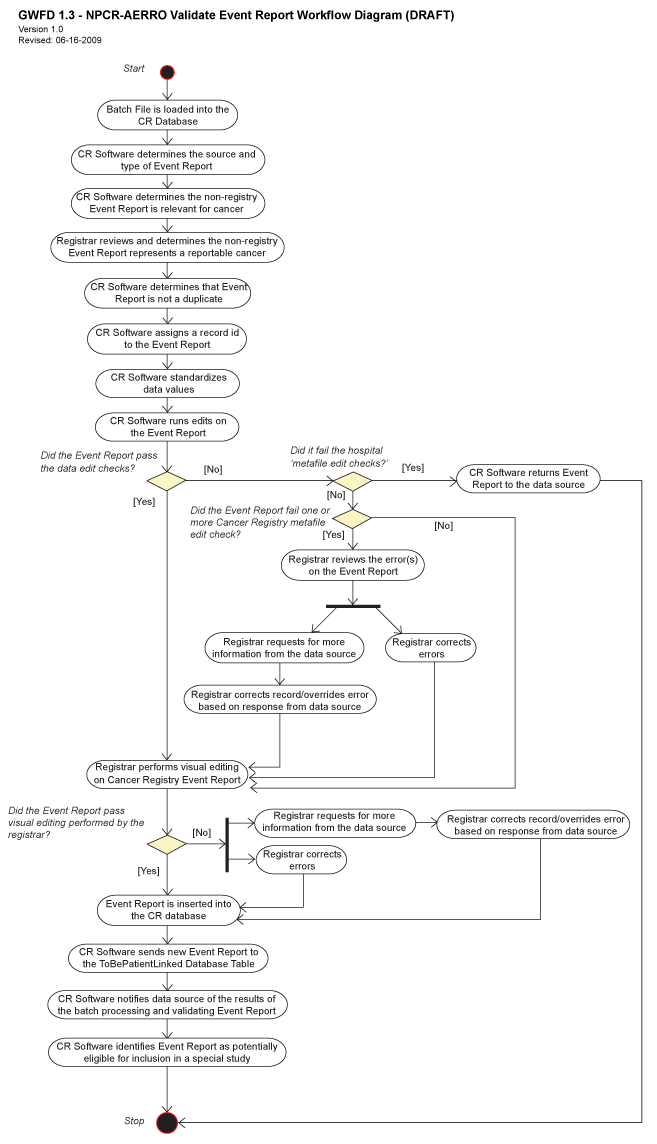
Hospital Validate Event Report Workflow Diagram
The validate event report workflow diagram shows the overall procedural flow of the functions. A text description of the diagram and legend may be found below. For information about reading diagrams, see Diagram Conventions.
Workflow Diagram Legend
The Validate Event Report use case begins after the Receive Batch File process has been performed and the event report is in the hospital and central cancer registry's active database tables.
The cancer registry (CR) reviews each event report to determine the type of event report. The CR determines whether the non-registry event report is relevant and reportable. Non-relevant and non-reportable event reports are deleted. The CR also standardizes certain data items on the non-registry event report, such as correcting the spelling of a city name and converting non-standard local codes to the NAACCR standards.
The CR determines whether the event report is a duplicate of a previously submitted event report. Duplicate event reports are deleted. Reportable, non-duplicate event reports are assigned a unique record ID. The record ID is never updated and cannot be re-assigned.
The CR performs a series of data validation checks, called edits, to verify the quality of the data in the event report. The CR reviews any event report failing these edits, correcting or overriding the edit as appropriate. The CR may request additional information from the data source to resolve the edit failure.
The CR may perform visual editing of the event report, comparing the text description with the coded data items to ensure accuracy.
The CR notifies the data source of the results of processing the event reports in a batch; this information includes the numbers of records submitted, duplicates, rejected event reports, and event reports with edit failures.
The CR notifies the special study group if an event report meets their eligibility criteria, and the use case ends. Processing continues with the Perform Patient Linkage Use Case.
- Page last reviewed: January 13, 2016
- Page last updated: January 13, 2016
- Content source:
- Maintained By:


 ShareCompartir
ShareCompartir