Hospital Submit Data Data Flow Diagram
The hospital submit data to the central cancer registry and the National Cancer Data Base data flow diagram shows the detailed procedural flow of control of the function. A text description of the diagram and legend may be found below. For information about reading diagrams, see Diagram Conventions.
- Step 1
Step 1. The registrar decides to report cases to a registry, agency, or project. - Steps 2 to 4
Step 2. The registrar selects the registry, agency, or project to which abstracts will be submitted. (BR01) Step 3. The cancer registry (CR) software selects abstracts that meet the reporting criteria. Step 4. The CR software runs validation checks. (SR01) - Step 5
Step 5. The CR software creates and transmits a batch file of eligible abstracts electronically. If the file cannot be transmitted electronically, the registrar transmits the batch file manually and the process continues with step 5.5. - Steps 6 to 8
Step 6. The CR software updates the data item Date Care Report Exported in the abstract. Step 7. The CR software updates the AbstractTransmissionHistory table. If the software cannot perform this update automatically, the registrar updates the AbstractTransmissionHistory Table manually. (BR02) Step 8. The process ends.
Data Flow Diagram Legend
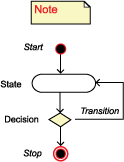
There are two actors: the registrar and the cancer registry (CR) software.
The process starts when the registrar decides to report cases to a registry, an agency, or a project. The registrar selects the registry, agency, or project to which the abstracts will be submitted.
The CR software selects abstracts that meet the criteria for reporting, runs validation checks on them, and creates a batch file of eligible abstracts.
If an electronic update of the database table is possible, the CR software transmits the batch file electronically. Otherwise, the registrar transmits it manually. The CR software then updates the data item "Date Case Report Exported" within the abstract.
If an electronic update of database table is possible, the CR software updates the AbstractTransmissionHistory table. Otherwise, the registrar updates the table manually and the process stops.
Business Rules (BR)
For definitions of the business rules and software requirements, please refer to the Submit Data to the Central Cancer Registry and the National Cancer Data Base Use Case [PDF-231KB].
- BR01 and BR02 apply to the decision to report cases, and selecting the cases to be reported based on criteria.
- BR03 applies to the process of updating database tables.
Software Requirement (SR)
- SR01 applies to running validation checks and creating a batch file.
- Page last reviewed: January 12, 2016
- Page last updated: January 12, 2016
- Content source:
- Maintained By:


 ShareCompartir
ShareCompartir