Side effects of cyproterone acetate
The side effects of cyproterone acetate (CPA), a steroidal antiandrogen and progestin, including its frequent and rare side effects, have been studied and characterized. It is generally well-tolerated and has a mild side-effect profile, regardless of dosage, when it used as a progestin or antiandrogen in combination with an estrogen such as ethinylestradiol or estradiol valerate in women.[1][2] Side effects of CPA include hypogonadism and associated symptoms such as demasculinization, sexual dysfunction, infertility, and osteoporosis; breast changes such as breast tenderness, enlargement, and gynecomastia; emotional changes such as fatigue and depression; and other side effects such as vitamin B12 deficiency, weak glucocorticoid effects, and elevated liver enzymes.[3] Weight gain can occur with CPA when it is used at high doses.[4][5] Some of the side effects of CPA can be improved or fully prevented if it is combined with an estrogen to prevent estrogen deficiency.[6][7] Few quantitative data are available on many of the potential side effects of CPA.[8] Pooled tolerability data for CPA is not available in the literature.[9]
At very high doses in aged men with prostate cancer, CPA can cause cardiovascular side effects. Rarely, CPA can produce blood clots, liver damage, excessively high prolactin levels, prolactinomas, and meningiomas. Upon discontinuation from high doses, CPA can produce adrenal insufficiency as a withdrawal effect.
| Side effect | Males (136 mg/day)[lower-alpha 1] (n = 1248) (%) | Females (18 mg/day)[lower-alpha 1] (n = 1258) (%) | Total (77 mg/day)[lower-alpha 1] (n = 2506) (%) |
|---|---|---|---|
| Elevated liver enzymes[lower-alpha 2] | 12.5 | 3.6 | 9.6 |
| Gynecomastia, breast pain | 4.9 | 1.0 | 2.9 |
| Overweightness, weight gain | 0.9 | 4.1 | 2.5 |
| Headache, migraine | – | 3.4 | 1.7 |
| Depression, anxiety, nightmares, mood swings | 1.0 | 1.8 | 1.4 |
| Gastrointestinal dysfunction | 0.2 | 2.3 | 1.2 |
| Thyroid dysfunction | 0.2 | 1.8 | 1.0 |
| Skin changes (pigmentation, chloasma, others) | 0.3 | 1.4 | 0.8 |
| Edema | – | 1.1 | 0.6 |
| Adrenal insufficiency or hyperplasia | 0.2 | 0.8 | 0.5 |
| Tiredness, lethargy, apathy | 0.6 | 0.6 | 0.6 |
| Alopecia, hair growth | 0.2 | 0.2 | 0.2 |
| Increased asthma attack rates | – | 0.3 | 0.2 |
| Osteoporosis | 0.2 | 0.08 | 0.2 |
| More frequently prone to attacks | 0.2 | 0.2 | 0.2 |
| Diabetes mellitus | 0.08 | 0.08 | 0.08 |
| Total (excluding elevated liver enzymes) | 9.0 | 19.1 | 14.0 |
| Notes: Data from the SExual-HOrmonal Surveillance STudy (SEHOST), a pharmacoepidemiologic active surveillance open-label uncontrolled follow-up study with historic (retrospective) accrual of patients. The sample included males and females aged 3 to 75 years with precocious puberty, hyperandrogenism, sexual deviance, and transgenderism; prostate cancer was not included. Sources: See template. | |||
| Side effect | Estradiol undecylate 100 mg/month i.m. (n = 96) | Cyproterone acetate 100 mg/day oral (n = 95) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Gynecomastia* | 74 | 77.1% | 12 | 12.6% |
| Breast tenderness* | 84 | 87.5% | 6 | 6.3% |
| Sexual impotence | "Occurred in essentially all patients of both groups" | |||
| Leg edema* | 17 | 17.7% | 4 | 4.2% |
| Thrombosis | 4 | 4.2% | 5 | 5.3% |
| Cardiovascular mortality | 2 | 2.1% | 2 | 2.1% |
| Other mortality | 1a | 1.0% | 0 | 0% |
| Notes: For 6 months in 191 men age 51 to 88 years with prostate cancer. Footnotes: * = Differences in incidences between groups were statistically significant. a = Due to unknown causes. Sources: See template. | ||||
Low hormone levels
Side effects in men resulting from the antiandrogenic and antigonadotropic properties of CPA include physical demasculinization, sexual dysfunction (including loss of libido and erectile dysfunction), absence of ejaculate, impaired spermatogenesis, testicular atrophy, and reversible infertility.[10][11] CPA has been described as causing "severe" suppression of sex drive and erectile potency in men with prostate cancer, comparable to that seen with surgical castration.[12] Due to suppression of the production of estrogens, long-term use of high-dose CPA without concomitant estrogen therapy can result in the development of osteoporosis in both sexes.[13] CPA can also sometimes cause breast changes in men including gynecomastia (breast development) and breast tenderness.[10] Rates of gynecomastia of 6 to 30% have been reported.[14][15][16][9] Galactorrhea (milk outflow) can also occur in men, due to the strong progestogenic effects of CPA.[10] The use of CPA in men has been associated with cellulite, which has been attributed to androgen deficiency as well.[17]
Depression
CPA has occasionally been associated with depressive mood changes in both men and women.[18] Similar depressive changes have been observed with castration, and may be a consequence of androgen deprivation in men.[18] In large studies, the incidence of depression has been 1 to 10% in both women taking high-dose CPA in combination with an estrogen for androgen-dependent skin and hair conditions and men taking high-dose CPA alone or in conjunction with castration for prostate cancer.[3][19][20] In some small studies however, relatively high rates of depression of 20 to 30% have been reported with both low- and high-dose CPA in combination with an estrogen in women.[21][22][23] In one large randomized controlled trial that performed a direct head-to-head comparison of high-dose (100 mg/day) versus low-dose CPA (2 mg/day), both in combination with an estrogen, the overall rate of depression was 12.7% and the rates did not differ between the two groups.[5] Despite some association with depression, the incidence of depression in women taking CPA in combination with an estrogen has been described by some researchers as "remarkably low", which they have said may be related to the positive psychological effects of the improvement in androgenic symptoms.[3]
A randomized controlled trial comparing the cognitive and emotional effects of GnRH agonists and CPA in 82 men with prostate cancer found no significant differences in scores on the Depression Anxiety Stress Scale-21 (DASS-21) after 6 months of treatment.[24] However, a 12-month follow-up of 62 of the men found a significant increase in emotional distress as measured by the DASS-21 in the CPA and watchful waiting groups relative to the GnRH-agonist groups.[25][26] Nonetheless, the mean levels of emotional distress remained within the normal range.[26]
A retrospective study reported that the rate of depression was greater with CPA (8.3%) than with GnRH analogues (2.2%) when both were used in combination with an estrogen in transgender women, although this study did not control for mood-related confounds.[27] Another retrospective study in transgender women, which used the Beck Depression Inventory‐II and other scales, found no significant differences in psychological well‐being or satisfaction with the combination of an estrogen and CPA or a GnRH agonist.[28] Hormone therapy in transgender women, including studies that used CPA, has been found to result in a significant decrease in depressive symptoms.[29][30]
In a series of relatively small studies of the combination of low-dose CPA and ethinylestradiol as a birth control pill, depression was reported to have occurred in 1.3 to 4% of cycles.[31] This is similar to the rate of mood changes (<3.5%) observed with birth control pills containing other progestins.[31] A pharmacoepidemiological study using data from the United Kingdom General Practice Research Database found that the incidence of depression with birth control pills containing CPA was identical to that of birth control pills containing other progestins (IRR = 0.99).[32] In clinical studies of the combination of low-dose CPA and estradiol valerate for the treatment of menopausal symptoms, preexisting adverse mood symptoms have been found to be significantly improved.[33]
Because of the possible side effect of worsened depressive symptoms, it may be advisable to use CPA with caution in individuals with a history of depression, particularly if severe.[34]
Bone loss
Androgen deprivation therapy, with medications such as CPA or GnRH modulators or with orchiectomy, results in profound deficiency of both androgens and estrogens in men.[35][36] These hormones, particularly estrogens, are known to be importantly involved in maintaining bone mineral density, both in men and women.[35][37] As a result, androgen deprivation therapy causes a rapid decrease in bone mineral density in men and can result in osteopenia or osteoporosis with long-term therapy.[35][36]
The bone loss that happens with androgen deprivation therapy typically occurs in the spine, hip, and forearm, but can also occur in other areas of the appendicular skeleton such as the femoral neck.[35] It can be seen within one year of treatment, and continues thereafter.[35] Lumbar spine bone density has been found to decrease at a rate of 3 to 7% per year with androgen deprivation therapy in men.[35] Androgen deprivation therapy also decreases muscle mass, and this may increase the risk of falls that can result in bone fractures as well as directly decrease bone mass.[35] The risk of bone fractures in men with prostate cancer given androgen deprivation therapy has been estimated to be increased by 45%.[35] The incidence of osteoporosis increases in men with prostate cancer from 36% to 80% after 10 years of androgen deprivation therapy, and following that, it can be expected that all treated men will develop osteopenia or osteoporosis.[38] The bone loss that occurs with androgen deprivation therapy in men is similar to that which occurs in postmenopausal women.[35]
Although androgen deprivation therapy is known to cause osteoporosis, it is thought that CPA may have a lower risk of osteoporosis than GnRH modulators or orchiectomy.[39] This is believed to be due to the progestogenic activity of CPA, which may help to inhibit bone resorption caused by sex-hormone deficiency.[39] However, the notion that progestogenic activity has beneficial effects on bone health is controversial.[40] Regardless of whether the notion is correct or not, case reports and case series of osteoporosis with CPA therapy in men have been published.[39][36] In addition, the use of progestogen-only birth control in women, with progestins similar to CPA like medroxyprogesterone acetate, has been found to result in decreased bone mineral density in premenopausal women.[41][42]
Calcium and vitamin D supplementation may help to reduce the risk of osteoporosis with androgen deprivation therapy in men.[35][38] Bisphosphonates can be used to reduce bone loss and fractures due to androgen deprivation therapy.[35][38] Estrogen replacement and selective estrogen receptor modulators may also be employed, although estrogens can produce gynecomastia in men and may increase the risk of male breast cancer.[35][38][37] Selective estrogen receptor modulators have been found to decrease the rate of bone loss due to androgen deprivation therapy in men by 50% after 2 years of therapy.[38] However, selective estrogen receptor modulators are described as substantially less effective for this indication than estrogens, which are the most effective medications for this purpose known.[43]
High progestogenic exposure
High prolactin levels
CPA increases prolactin levels and can produce hyperprolactinemia (high prolactin levels) due to its progestogenic activity and consequent stimulation of pituitary lactotrophs.[44][45][46][47][48][49][50][51][52][53] Increases in prolactin levels can occur at low, medium, and high doses of CPA, with or without an estrogen.[3] Elevation of prolactin levels may be greater and hyperprolactinemia more likely to occur when CPA is combined with an estrogen, as combinations of estrogens and progestogens show synergistic increases in prolactin levels in non-human primates.[54][55][56] Although hyperprolactinemia can develop with CPA, prolactin levels only rarely exceed the normal physiological range, and increased prolactin levels with CPA are said to be seldom of clinical significance.[3]
At a dosage of 10 mg/day in men, CPA has been found to increase prolactin levels by 75%.[57][58][59] In another study, a combination of 2 or 20 mg/day CPA with testosterone undecanoate resulted in modest increases in prolactin levels (+96%) in men similarly.[60] The increase in prolactin levels in men with 100 mg/day oral CPA (+118%) has been found to be less than that with 100 mg/month intramuscular estradiol undecylate (+192%).[45] The combination of high-dose (100 μg/day) ethinylestradiol and high-dose (100 mg/day) oral CPA in transgender women has been reported to increase the risk of hyperprolactinemia by 425-fold (20% incidence) relative to cisgender men.[61] However, other studies using the same regimen have found lower incidences of hyperprolactinemia (e.g., 4–7% per year).[62][63] In one study, prolactin levels were 18 ng/mL at the start of treatment, 23 ng/mL after 3 months of 100 mg/day CPA, and 19 to 28 ng/mL after 6 to 15 months of 100 μg/day ethinylestradiol and 100 mg/day oral CPA in transgender women.[63]
The major symptom of hyperprolactinemia as a condition is hypogonadism, which is caused by the antigonadotropic effects of prolactin.[53] Symptoms consequent to hypogonadism include amenorrhea, menopausal symptoms like hot flashes and vaginal dryness, sexual dysfunction, infertility, reduced muscle mass, and decreased bone mineral density.[53][64][65] However, due to its progestogenic actions and the potent antigonadotropic effects produced by this activity, CPA can produce such symptoms as side effects regardless of the presence or absence of hyperprolactinemia.[11][35][13]
Prolactin additionally has a direct inhibitory effect on sexual desire in men that is independent of hypogonadism.[66][67][68] In men with hyperprolactinemia and hypogonadism, sexual desire is not restored by testosterone replacement therapy, but is restored by prolactin-suppressing medications.[66][67] Conversely, erectile dysfunction in men with hyperprolactinemia is considered to be due to hypogonadism rather than due to direct actions of prolactin itself.[66] On the other hand, it is thought that prolactin may be responsible for the normal sexual refractory period in men, and may directly inhibit the capacity for orgasm.[68]
In addition to symptoms of hypogonadism and sexual dysfunction, hyperprolactinemia can cause breast changes such as breast pain, breast enlargement, gynecomastia, and galactorrhea.[53][69][70] Galactorrhea secondary to hyperprolactinemia is very common in premenopausal women (80%) but more uncommon in individuals with low estrogen levels such as postmenopausal women and men.[69] Gynecomastia likewise occurs at a relatively low rate in men with hyperprolactinemia (23%).[69] A study in transgender women found that galactorrhea occurred in 7% treated with 100 μg/day ethinylestradiol and 100 mg/day oral CPA.[63]
Prolactin increases levels of adrenal androgens like dehydroepiandrosterone and dehydroepiandrosterone sulfate by increasing their production and secretion from the adrenal glands.[71][72][73][74] For this reason, hyperprolactinemia can cause symptoms of hyperandrogenism such as acne and hirsutism in women.[53][75] However, such symptoms are not described with CPA, which instead treats these conditions; this can be attributed to the antiandrogenic and glucocorticoid activity of CPA.[3] Through such activities, CPA directly blocks the actions of androgens and can suppress dehydroepiandrosterone sulfate secretion from the adrenal glands.[3][76][77][7]
Benign brain tumors
The combination of an estrogen and CPA has been associated, albeit rarely, with the incidence and/or aggravation of a few different types of benign brain tumors, most notably prolactinomas and meningiomas.[78][79] Prolactinomas are benign tumors of the pituitary gland that secrete prolactin, and are one of several types of secreting pituitary adenomas.[63][61] They can produce hyperprolactinemia (abnormally high prolactin levels) as a symptom, which often leads to their diagnosis.[63][61] Meningiomas are usually-benign tumors of the meninges, the membranes that envelop the brain and spinal cord.[80][81] Due to its association with meningiomas, CPA is considered to be contraindicated in people with meningioma or a history of meningioma.[82][34] Benign brain tumors associated with an estrogen and/or CPA can cause visual disturbances or in severe cases complete blindness due to compression of the optic nerve and/or chiasm.[78]
There is expression of the PR in the anterior pituitary and high expression of the PR in the meninges, suggesting that activation of the PR by CPA is involved in the pathogenesis of the benign brain tumors associated with it.[83] Estrogen receptors are also expressed in the anterior pituitary,[84] and are known to increase the expression of the PR in this area.[85] In accordance, estrogens and progestogens, including CPA, are known to increase prolactin levels, particularly at high concentrations (e.g., pregnancy).[51][86] While most cases of benign brain tumors associated with CPA have been in combination with an estrogen and have occurred in transgender women, there have been cases associated with high-dose CPA alone in both cisgender women and men as well.[54][87] Benign brain tumors such as meningiomas have also been associated with high dosages of other progestogens, such as chlormadinone acetate, megestrol acetate, and medroxyprogesterone acetate, as well as with pregnancy.[88][89][90][91]
A number of case reports of prolactinomas (as well as a non-secreting pituitary adenoma),[92][93][94][95][96] meningiomas,[97][98][99][100][89][87][78] and vestibular schwannomas[79] associated with estrogen and CPA therapy in transgender women have been published.[101][102][103][79][88][104] A large retrospective chart study of 2,555 transgender women treated most frequently with an estrogen and CPA and followed for 23,935 person-years reported occurrences of 8 meningiomas (0.31% or 1 in 320 incidence; SIR = 4.1 relative to cisgender females, SIR = 11.9 relative to cisgender males), 9 prolactinomas (0.35% or 1 in 284 incidence; SIR = 4.3 relative to cisgender females, SIR = 26.5 relative to cisgender males), one non-secreting pituitary adenoma, and two vestibular schwannomas.[79] A retrospective study of 303 transgender women treated with high-dose estrogen and CPA and followed for a median 4.4 years (range 6 months to 6 months to 14 years) reported occurrences of 46 cases of hyperprolactinemia (serum prolactin >1,000 mU/L) (15% incidence; 400-fold increased risk relative to cisgender males).[61][62][103][63][105] Of 23 persistent cases, 15 of which returned for follow up, 5 of the cases showed an enlarged pituitary gland on CT scanning with contrast enhancement.[61] Dosages of CPA in combination with an estrogen that have been associated with benign brain tumors have been 10 mg/day and above.[79][62][104][87] In contrast to the combination of an estrogen and CPA, estrogen alone has been associated only with single case reports of prolactinomas in transgender women.[105][106][79]
A nationwide population study in Denmark found no significant association of CPA or other antiandrogens with meningioma in men.[107] However, studies in Spain and the United Kingdom have found positive associations of CPA therapy with meningioma.[107]
| # | Age | Sex | Medications | Treatment duration | Ref | Link | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 26 years | MtF | CPA 100 mg/day, EE 100 μg/day, EU 100 mg/2x week | ~10 months | Gooren et al. (1988) | [92] | ||
| 2 | 32 years | MtF | CPA 150 mg/day, EE 1.5 mg/day | 4 years | Serri et al. (1996) | [93] | ||
| 3 | 52 years | MtF | CPA 100 mg/day, EE 100 μg/day | 15 years | Bunck et al. (2009) | [95] | ||
| 4 | 33 years | MtF | CPA 200 mg/day, CEEs 2.5 mg/day | 6 months | García-Malpartida et al. (2010) | [94] | ||
| 5 | 41 years | MtF | CPA 2 mg/day, EE 35 μg/day, E2-EN 10 mg/2 weeks i.m. | 18 years | Cunha et al. (2015) | [96] | ||
| 6 | 32 years | MtF | CPA 100 mg/day, E2 injections 100 mg/2 weeks i.m. | 53 months | Nota et al. (2018) | [79] | ||
| 7 | 39 years | MtF | CPA 100 mg/day, CEEs 2.5 mg/day | 172 months | Nota et al. (2018) | [79] | ||
| 8 | 27 years | MtF | CPA, E2 injections i.m. (no dosage information) | 156 months | Nota et al. (2018) | [79] | ||
| 9 | 46 years | MtF | CPA 100 mg/day, EE 100 μg/day | 66 months | Nota et al. (2018) | [79] | ||
| 10 | 24 years | MtF | CPA 100 mg/day (no estrogen mentioned) | 9 months | Nota et al. (2018) | [79] | ||
| 11 | 47 years | MtF | CPA 100 mg/day, EE 100 μg/day | 91 months | Nota et al. (2018) | [79] | ||
| 12 | 28 years | MtF | CPA 50 mg/day, EV 2 mg/day oral | 134 months | Nota et al. (2018) | [79] | ||
| 13a | 34 years | MtF | CPA 50 mg/day, EV 1 mg/day oral | 35 months | Nota et al. (2018) | [79] | ||
| Abbreviations: CPA = Cyproterone acetate. E2 = Estradiol. EV = Estradiol valerate. E2-EN = Estradiol enanthate. EU = Estradiol undecylate. CEEs = Conjugated estrogens. EE = Ethinylestradiol. MtF = Male-to-female (transgender woman). i.m. = Intramuscular injection. Footnotes: a = Non-secretive pituitary adenoma. Notes: Asscheman et al. (1988) also described 5 MtF cases of pituitary enlargement and possible prolactinoma.[62] van Kesteren et al. (1997) described possible MtF cases of pituitary enlargement as well.[108] Five estrogen-only MtF cases (without CPA) have also been reported.[106][95][96][79] Futterweit (1998) described an MtF case without information about medications.[109] Sources: [104] | ||||||||
| # | Age | Sex | Medications | Treatment duration | Ref | Link | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 28 years | MtF | CPA 100 mg/day, EE 100 μg/day | 5 years | Gazzeri et al. (2007) | [97] | ||
| 2 | 48 years | MtF | CPA 100 mg/day, EE ("feminizing regimen") | 10 years | Cebula et al. (2010) | [99] | ||
| 3 | 46 years | Female | CPA 50 mg/day, E2 ("substitutive") | 10 years | Gonçalves et al. (2010) | [110] | ||
| 4 | 83 years | Male | CPA ≥50 mg/day | 2 years | Gil et al. (2011) | [54] | ||
| 5 | 71 years | Male | CPA ≥50 mg/day | 3 years | Gil et al. (2011) | [54] | ||
| 6 | 66 years | Female | CPA ≥50 mg/day | 3 years | Gil et al. (2011) | [54] | ||
| 7 | 43 years | Female | CPA ≥50 mg/day | 2 years | Gil et al. (2011) | [54] | ||
| 8 | 35 years | MtF | CPA 100 mg/day, E2 100 μg/day patch | 4 years | Bergoglio et al. (2012) | [100] | ||
| 9 | 60 years | MtF | CPA 50 mg/day, E2 8 mg/day oral | 10 years | Knight et al. (2013) | [111] | ||
| 10 | 56 years | MtF | CPA, EV 2 mg/day oral | 8 years | Razavi (2014) | [112] | ||
| 11 | 51 years | Female | CPA 15 mg/day | 30 years | Bernat et al. (2015) | [113] | ||
| 12 | 47 years | Female | CPA 25 mg/day | 15 years | Bernat et al. (2015) | [113] | ||
| 13 | 43 years | Female | CPA 50 mg/day | 12 years | Bernat et al. (2015) | [113] | ||
| 14 | 39 years | Female | CPA 50 mg/day | 10 years | Bernat et al. (2015) | [113] | ||
| 15 | 61 years | Female | CPA 25 mg/day | 24 years | Bernat et al. (2015) | [113] | ||
| 16 | 38 years | Female | CPA 25 mg/day | 10 years | Bernat et al. (2015) | [113] | ||
| 17 | 45 years | Female | CPA 50 mg/day | 20 years | Bernat et al. (2015) | [113] | ||
| 18 | 53 years | Female | CPA 50 mg/day | 20 years | Bernat et al. (2015) | [113] | ||
| 19 | 56 years | Female | CPA 50 mg/day | 8 years | Bernat et al. (2015) | [113] | ||
| 20 | 55 years | Female | CPA 50 mg/day | 30 years | Bernat et al. (2015) | [113] | ||
| 21 | 49 years | Female | CPA 50 mg/day | 20 years | Bernat et al. (2015) | [113] | ||
| 22 | 49 years | Female | CPA 50 mg/day | 25 years | Bernat et al. (2015) | [113] | ||
| 23 | 58 years | Female | CPA 50 mg/day | 18 years | Botella et al. (2015) | [114] | ||
| 24 | 37 years | Female | CPA 50 mg/day | 11 years | Botella et al. (2015) | [114] | ||
| 25 | 42 years | Male | CPA 100 mg/day | 23 years | Sys & Kestelyn (2015) | [115] | ||
| 26 | 45 or 46 years | MtF | CPA 10 or 100 mg/day, E2 20 mg/4 months implant | 5 years | ter Wengel et al. (2015) | [89][79] | ||
| 27 | 51 years | MtF | CPA 100 mg/day, EE 100 μg/day | 25 years | ter Wengel et al. (2015) | [89][79] | ||
| 28 | 65 years | MtF | CPA 10 mg/day, CEEs 1.25 mg/day | 19 years | ter Wengel et al. (2015) | [89][79] | ||
| 29 | 26 years | Female | CPA 50 mg/day | 10 years | Kalamarides & Peyre (2017) | [116] | ||
| 30 | 43 years | Female | CPA 25 mg/day | 25 years | Kalamarides & Peyre (2017) | [116] | ||
| 31 | 83 years | Male | CPA 200 mg/day | 7 months | Keilani & Abada (2017) | [117] | ||
| 32 | 77 years | MtF | CPA 50 mg/day, E2 50 μg/day patch | 24 years | Boer et al. (2018) | [118] | ||
| 33 | 41 years | MtF | CPA 50 mg/day, E2 gel 1–3 mg/day | 9 years | Mancini et al. (2018) | [87] | ||
| 34 | 51 years | MtF | CPA 20 mg/week, E2 100 μg/day patch | 11 years | Nota et al. (2018) | [79] | ||
| 35 | 66 years | MtF | CPA 10 mg/day | 40 years | Nota et al. (2018) | [79] | ||
| 36 | 58 years | MtF | CPA 50 mg/day, E2 100 μg/day patch | 6 years | Nota et al. (2018) | [79] | ||
| 37 | 49 years | MtF | CPA, EV 2 mg/day | 16 years | Nota et al. (2018) | [79] | ||
| 38 | 51 years | MtF | CPA 100 mg/day, EE 100 μg/day patch | 26 years | Nota et al. (2018) | [79] | ||
| 39 | 50 years | MtF | CPA 50 mg/day, E2 0.6 mg/g cream 2x/day | ~10 years | Raj et al. (2018) | [78] | ||
| 40 | 48 years | MtF | CPA 50 mg/day, E2 1 mg/g cream 3x/day | 21 years | Raj et al. (2018) | [78] | ||
| Abbreviations: CPA = Cyproterone acetate. E2 = Estradiol. EV = Estradiol valerate. CEEs = Conjugated estrogens. EE = Ethinylestradiol. MtF = Male-to-female (transgender woman). Notes: For the Bernat et al. (2015) cases, only one was reported to be taking an estrogen (specifically estradiol).[113] Froelich et al. (2008) reported an additional 8 female cases (age 33–62 years, mean 46 years; 50 mg/day CPA; 10–20 years exposure) with multiple meningiomas.[119] Cea-Soriano et al. (2012) also reported 8 cases (4 male (≥50 mg/day, OR = 3.28), 4 female (2 mg/day, OR = 1.03)) with no individual specifics.[102][114] Cases in association with other progestins have been reported as well.[113] Deipolyi, Han, & Parsa (2010) reported a case in an MtF in association with E2 100 μg/day only.[98] Sources: [87][89][104] | ||||||||
Blood clots
The combination of low-dose (2 mg) CPA in combination with ethinylestradiol (35 μg), as in combined birth control pills, presents an increased risk of venous thromboembolism (VTE).[120][121] Women who take contraceptive pills containing CPA have a 6- to 7-fold increased risk of developing VTE compared to women not taking a contraceptive pill, and twice the risk of women who take a contraceptive pill containing the androgenic progestin levonorgestrel.[122] The absolute risk of VTE with ethinylestradiol and low-dose CPA-containing birth control pills is about 1.2 to 9.9 per 10,000 women-years.[121] At least four cases of fatal VTE have been attributed to birth control pills containing ethinylestradiol and low-dose CPA.[123] The progestogenic, antiandrogenic, and glucocorticoid activities of CPA are all thought to be involved in the increased risk of VTE with CPA in combination with estrogens.[124][125]
The combination of oral 100 μg/day ethinylestradiol and 100 mg/day CPA was reported to produce a 45-fold increase in the risk of VTE in 303 transgender women, with an absolute incidence of 6.3% (19 cases).[126] The risk was highly age-dependent, with a rate of VTE of 2.1% in those less than 40 years of age and of 12% in those over 40 years of age.[126] In a subsequent study of 816 transgender women in whom the same regimen was used but transdermal estradiol had become the standard therapy for those over the age of 40, the risk of VTE was still increased overall by 20-fold (45 cases, 5.5% incidence).[126] However, there was only a single case of VTE in the group of 138 transgender women treated with transdermal estradiol (0.7% incidence).[126] In accordance, the combination of transdermal estradiol and 50 mg/day cyproterone acetate appears to be relatively safe in terms of VTE risk.[126] The VTE risk was initially attributed exclusively to ethinylestradiol, and the use of ethinylestradiol has largely been abandoned in transgender women in favor of other estrogens such as estradiol because of it.[126] However, CPA is now known to significantly increase the risk of VTE as well, and it may have contributed also.[126] CPA should be discontinued in transgender women after sex reassignment surgery or orchiectomy to reduce the risk of VTE.[126] It should also be discontinued at least 2 weeks before undergoing surgery to reduce the risk of VTE.[126]
A large pharmacoepidemiological study in the United Kingdom using the General Practice Research Database assessed the risk of VTE with various forms of androgen deprivation therapy for prostate cancer.[127][128][129] The study had a sample of 11,199 men, of whom 229 (2.0%) experienced VTE and in whom 14% this was fatal.[127][129] The incidence rates for VTE were 3.46 for CPA monotherapy relative to nonsteroidal antiandrogen monotherapy with flutamide or bicalutamide; 3.35 for CPA monotherapy relative to GnRH agonist/orchiectomy monotherapy; 1.25 for CPA monotherapy relative to estrogen monotherapy with diethylstilbestrol or estramustine phosphate; and 0.60 for CPA monotherapy relative to combined androgen blockade with a GnRH agonist/orchiectomy and CPA.[128][8] The adjusted odds ratios for VTE were 1.00 for no treatment; 1.29 for nonsteroidal antiandrogen therapy; 3.35 for combined androgen blockade with CPA and a GnRH agonist/orchiectomy; 5.23 for CPA monotherapy; and 5.67 for estrogen monotherapy.[127][129][130] The adjusted odds ratios for VTE of different dosages of CPA with or without a GnRH agonist relative to GnRH agonist monotherapy were 3.49 for 25 or 50 mg/day, 4.93 for 100 or 150 mg/day, and 4.54 for greater than or equal to 200 mg/day.[129] In addition to CPA and other medications used to treat prostate cancer, metastatic prostate cancer is itself a risk factor for VTE.[126]
In large randomized controlled trials of CPA versus other medications for the treatment of prostate cancer, the following incidences of VTE have been observed: 5.3% for 100 mg/day oral CPA vs. 4.2% for 100 mg/month intramuscular estradiol undecylate (n = 191);[131][132] 2.4% for 250 mg/day oral CPA vs. 6.1% for 200 mg/day oral medroxyprogesterone acetate vs. 8.2% for 3 mg/day oral diethylstilbestrol (n = 269);[133] and 4.5% for 300 mg/day oral CPA vs. 0% for 750 mg/day flutamide (n = 264).[133][134] However, the final analysis of the last study (EORTC Protocol 30892) indicated that VTE ultimately ended up occurring in 3 patients (2.0%) in the flutamide group and 7 patients (4.6%) in the CPA group.[16]
Premenopausal women using depot injectable medroxyprogesterone acetate, a progestin related to CPA, as a form of progestogen-only birth control, have been observed to have a 2.2- to 3.6-fold increased risk of VTE.[135][136] However, this could have reflected preferential prescription of DMPA to women considered to be at an increased risk of VTE.[136] DMPA has little or no effect on coagulation and fibrinolytic factors.[137][138] In addition, progestogens by themselves normally do not increase the risk of thrombosis.[136][139]
Cardiovascular effects
CPA is associated with an incidence of relatively mild cardiovascular side effects when it is used at high doses to treat prostate cancer in aged men.[140][141][142][143][144] These include coagulation changes[145] and blood clots (5%),[140][146] fluid retention (4%),[146] ischemic cardiomyopathy (4–40%),[9][147] and undesirable effects on serum lipid profiles.[140][141][142][143][144] Severe cardiovascular complications occur in up to 10% at such doses and are sometimes fatal.[144][148] However, a large randomized controlled trial that compared CPA and flutamide in men with prostate cancer found that the rates of cardiovascular problems were not significantly different between the two therapies.[16] The cardiovascular toxicity of 250 mg/day CPA is significantly lower than that of 3 mg/day diethylstilbestrol.[133][149]
Long-term effects
The Women's Health Initiative and other clinical studies observed a significantly increased risk of breast cancer, blood clots, and cardiovascular disease when 2.5 mg/day medroxyprogesterone acetate, a progestin related to CPA, was added to 0.625 mg/day conjugated estrogens in postmenopausal women.[150][151] Similarly, the use of an estrogen and many other progestogens, including low-dose CPA, in menopausal hormone therapy has been found to be associated with a significantly higher incidence of breast cancer in postmenopausal women.[152]
In terms of ovulation inhibition, the effective dosage of CPA is 1.0 mg/day while that of medroxyprogesterone acetate is 10 mg/day.[124][153] Based on ovulation inhibition, a dosage of 50 mg/day cyproterone acetate has on the order of 200 times the progestogenic potency of 2.5 mg/day medroxyprogesterone acetate.[124][153] In addition to its progestogenic activity, CPA produces androgen and estrogen deficiency when used as a monotherapy,[154] and this influences health as well.[155][156][37]
The health effects of high-dose CPA with long-term therapy have not been well-studied. A meta-analysis of high-dose CPA for the treatment of prostate cancer in men found that CPA was associated with a slight excess of non-prostate cancer deaths.[157] In addition, the combination of CPA with surgical or medical castration for prostate cancer has been found to significantly decrease overall survival relative to castration alone.[158]
| Event | Relative Risk CEEs/MPA vs. placebo at 5.2 years (95% CI[note 1]) | Placebo (n = 8102) | CEEs/MPA (n = 8506) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute Risk per 10,000 Women-Years | |||||||||
| Coronary heart disease events ( non-fatal myocardial infarction, death) | 1.29 (1.02–1.63) 1.32 (1.02–1.72) 1.18 (0.70–1.97) | 30 23 6 | 37 30 7 | ||||||
| Invasive breast cancer[lower-alpha 3] | 1.26 (1.00–1.59) | 30 | 38 | ||||||
| Stroke | 1.41 (1.07–1.85) | 21 | 29 | ||||||
| Pulmonary embolism | 2.13 (1.39–3.25) | 8 | 16 | ||||||
| Colorectal cancer | 0.63 (0.43–0.92) | 16 | 10 | ||||||
| Endometrial cancer | 0.83 (0.47–1.47) | 6 | 5 | ||||||
| Hip fracture | 0.66 (0.45–0.98) | 15 | 10 | ||||||
| Death due to causes other than above | 0.92 (0.74–1.14) | 40 | 37 | ||||||
| Global Index[lower-alpha 4] | 1.15 (1.03–1.28) | 151 | 170 | ||||||
| Deep vein thrombosis[lower-alpha 5] | 2.07 (1.49–2.87) | 13 | 26 | ||||||
| Vertebral fractures[lower-alpha 5] | 0.66 (0.44–0.98) | 15 | 9 | ||||||
| Other osteoporotic fractures[lower-alpha 5] | 0.77 (0.69–0.86) | 170 | 131 | ||||||
| Sources: See template. | |||||||||
Other side effects
Liver toxicity
The most serious potential side effect of CPA is hepatotoxicity.[159] A variety of manifestations of liver disease in association with CPA treatment have been documented, including immunoallergic cytotoxic reactions, cholestasis, autoimmune hepatitis, acute hepatitis, fulminant liver failure, and cirrhosis, as well as an increased risk of hepatocellular carcinoma.[160][161] Clinical features may include jaundice, fatigue, nausea, elevated liver enzymes, hepatic necrosis and inflammation, and features of hepatic decompensation.[161] Hepatotoxicity due to CPA therapy is most common in elderly patients who are treated with high dosages of the drug for prolonged periods of time, but has also occurred in younger patients.[160] The hepatotoxicity of CPA is related to its C1α,2α methylene group.[76]
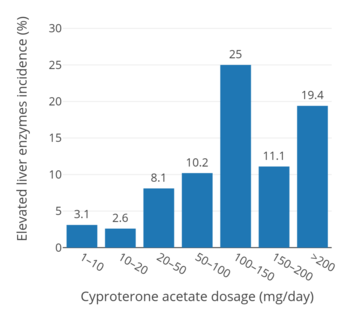
In an uncontrolled open-label active surveillance study of 1,685 healthy males and females of all ages (3 to 75 years for the full sample of 2,506 individuals) treated with CPA for an average of 6.7 years (but in 602 individuals for up to more than 10 years), elevated liver enzymes were seen in 2.6 to 3.1% of individuals at a dosage of 1 to 20 mg/day, in 8.1% of individuals at a dosage of 20 to 50 mg/day, in 10.2% of individuals at a dosage of 50 to 100 mg/day, and in 11.1 to 25.0% of individuals at a dosage of greater than 100 mg/day (up to more than 200 mg/day).[19][159][160][161] In a trial of 89 men with prostate cancer who received 50 mg/day CPA for 4 years, elevated liver enzymes occurred in 28.2%.[161][162] A study of 105 patients treated with 150 mg/day CPA reported a hepatotoxicity rate of 9.5%, with serious liver injury occurring in 3.8% (4/105).[161] A study of 303 transgender women treated with high-dose estrogen and 100 mg/day CPA reported an incidence of elevated liver enzymes of 7.2% (22/303).[61]
In 2002, it was reported that there were 18 published case reports of CPA-associated hepatitis in the literature, with 6 of these cases resulting in death.[159] In addition however, a 1995 publication by the United Kingdom Medicines Control Agency/Committee on Safety of Medicines in its journal Current Problems in Pharmacovigilance described an additional 96 spontaneous reports of hepatotoxicity (91 males, 5 females), with 33 of these cases resulting in death.[163][164][159] The manifestations of hepatotoxicity described in this publication included hepatitis, cholestatic jaundice, and liver failure.[163][164] The majority of cases were men being treated with very high doses of CPA (300 mg/day) for prostate cancer.[163][164] A 2014 review found that 9 cases specifically of CPA-induced fulminant (sudden-onset and severe) liver failure had been reported to date, with only one of these cases not being fatal.[161] As such, the prognosis of CPA-induced liver failure is death.[161] However, serious hepatotoxicity occurs mostly in prostate cancer patients who take very high doses of CPA, and serious liver toxicity has not been reported in transgender women.[165] All 14 reported cases of serious hepatotoxicity (acute liver failure and acute hepatitis) with CPA described in the 2014 review were in a dosage range of 100 to 300 mg/day and were in elderly men with prostate cancer (age range 65 to 92 years).[160] A 2015 publication reported an additional 22 new cases of hepatotoxicity in association with CPA, including one case at 50 mg/day.[166]
The risk of hepatotoxicity and death associated with CPA treatment is reportedly the reason that CPA has not been approved by the FDA for use in the United States.[167] Patients being treated with high-dose CPA should be closely monitored with liver function tests.[168] The risk is dose-dependent, and the low doses of CPA used in birth control pills (2 mg) have been said to represent a non-significant risk.[169] However, a German woman who had been taking Diane-35 (containing 2 mg/day CPA) for contraception for 14 years died of liver cancer, and this led to a safety review by drug regulators and the eventual restriction of CPA throughout Europe for the indication of acne treatment in women.[123] In any case, liver toxicity with CPA occurs mostly in prostate cancer patients who take very high doses of the medication (200–300 mg/day), and liver toxicity has not been reported in cisgender or transgender women, who usually take lower doses (25–100 mg/day).[160][165][6][7]
The hepatotoxicity of the nonsteroidal antiandrogen flutamide is greater than that of CPA.[16][170] In a randomized controlled trial and direct head-to-head comparison for prostate cancer, overall rates of liver function deterioration were 9.9% in the 750 mg/day flutamide group and 5.3% in the 300 mg/day oral CPA group (p = 0.128), while liver toxicity requiring discontinuation occurred in 8.6% in the flutamide group and 2.0% in the CPA group.[16] Findings were similar in another randomized controlled trial and direct head-to-head comparison of 750 mg/day flutamide and 150 mg/day oral CPA; the rates of hepatotoxicity were 15.3% for flutamide and 9.5% for CPA (p = 0.034) and the rates of serious hepatotoxicity were 4.8% for flutamide and 3.8% for CPA.[170] A 2004 review cited 46 published cases of hepatotoxicity in association with flutamide, 21 cases with CPA, 4 cases with nilutamide, and 1 case with bicalutamide, all between 1986 and 2003.[171][172]
There are case reports of suspected cross-hepatotoxicity between CPA and flutamide.[173][174]
| # | Sex | Age | Dosage | Type | Onset | Outcome | Survivala | Ref | Link |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 73 years | 400 mg/day | AH | 2.5 months | Survived | N/A | Meijers et al. (1986) | [175] |
| 2 | Female | 85 years | 200 mg/day | AH | 4.8 months | Survived | N/A | Meijers et al. (1986) | [175] |
| 3 | Male | 78 years | 200 mg/day | ALF | 6 months | Death | 2 weeks | Lévesque et al. (1989) | [176] |
| 4 | Male | 71 years | 300 mg/day | AH | 5.3 months | Survived | N/A | Blake et al. (1990) | [177] |
| 5 | Male | 79 years | 200–300 mg/day | AH | 2.5 months | Survived | N/A | Dore et al. (1990) | [178] |
| 6 | Male | 80 years | 200 mg/day | ALF | ND | Death | ? | Antoni et al. (1991) | [179] |
| 7 | Male | 75 years | 300 mg/day | HCC | 1.5 years | ND | ND | Ohri et al. (1991) | [180] |
| 8 | Male | 72 years | 300 mg/day | ALF | ND | Survived | N/A | Parys et al. (1991) | [181] |
| 9 | Male | 65 years | 300 mg/day | ALF | 1 year | Death | 1.6 months | Parys et al. (1991) | [181] |
| 10 | Male | 83 years | 300 mg/day | ALF | 1.25 years | Death | 2 weeks | Parys et al. (1991) | [181] |
| 11 | Male | 78 years | 150 mg/day | AH | ~3 months | Survived | N/A | Drakos et al. (1992) | [182] |
| 12 | Female | 24 years | 100 mg/day (RS) | CH | 3 months | Survived | N/A | Hassler et al. (1992) | [183] |
| 13 | Male | 74 years | 200 mg/day | AH | 11 months | Survived | N/A | Roila et al. (1993) | [184] |
| 14 | Male | 79 years | 300 mg/day | ALF | 10 months | Death | 2 weeks | Bressollette et al. (1994) | [185] |
| 15 | Male | 92 years | 100 mg/day | ALF | 4 months | Death | 5 weeks | Hirsch et al. (1994) | [186] |
| 16 | Male | 65 years | 600 mg/day | HCC | 4 months | Survived | N/A | Kattan et al. (1994) | [187] |
| 17 | Female | 22 years | 100–250 mg/day | HCC | 10 years | Death | 9 months | Watanabe et al. (1994) | [188][189] |
| 18 | Female | 19 years | 200–300 mg/day | HCC | 9 years | Survived | N/A | Watanabe et al. (1994) | [188][189] |
| 19 | Female | 19 years | 200 mg/day | HCC | ~10 years | Survived | N/A | Watanabe et al. (1994) | [188][189] |
| 20 | Male | 87 years | 200 mg/day | ALF | 4 months | Death | ~3.5 weeks | Pinganaud et al. (1995) | [190] |
| 21 | Male | 78 years | 150 mg/day | ALF | 1 year | Death | 3 weeks | Pinganaud et al. (1995) | [190] |
| 22 | Female | 45 years | 2 mg/day (BCP) | HCC | 14 years | Death | 9 months | Rüdiger et al. (1995) | [191] |
| 23 | Male | 78 years | 200–300 mg/day | ALF | 3 months | Death | 9 months | Castellani et al. (1996) | [192] |
| 24 | Male | 73 years | 300 mg/day | ALF | 4 months | Survived | N/A | Murphy et al. (1996) | [193] |
| 25 | Male | 64 years | 100 mg/day | AH | 6 months | Survived | N/A | Ruiz-Rebollo et al. (1997) | [194] |
| 26 | Female | ≥8 years | 200–300 mg/day | HCC | >4 years | Survived | N/A | Watanabe et al. (1997) | [189] |
| 27 | Male | 21 years | 100–350 mg/day | HCC | 15 years | Survived | N/A | Watanabe et al. (1997) | [189] |
| 28 | Male | 84 years | ND | ALF | ND | Death | 1 week | Lombardi et al. (1998) | [195] |
| 29 | Male | 81 years | 300 mg/day | ALF | 6 months | Death | 1.6 months | Friedman et al. (1999) | [196] |
| 30 | Male | 66 years | 300 mg/day | ALF | 2 months | Death | 4 weeks | Friedman et al. (1999) | [196] |
| 31 | Male | 14 years | 100 mg/day | Cirrhosis | ~7.5 years | Death | ~1 year | Garty et al. (1999) | [197] |
| 32 | Male | 84 years | 100–300 mg/day | HCC | 10 years | Death | 6 days | Manfredi et al. (2000) | [198] |
| 33 | Male | 87 years | 300 mg/day | AH | ND | Survived | N/A | Giordano et al. (2001) | [199] |
| 34 | Female | 17 years | 2 mg/day (BCP) | AIH/cirrhosis | 2 months | Survived | N/A | Kacar et al. (2002) | [200] |
| 35 | Male | 76 years | 150 mg/day | AH | 7 months | Survived | N/A | Manolakopoulos et al. (2004) | [174] |
| 36 | Male | 78 years | 200 mg/day | ALF | 3 months | Death | 1.0 months | Famularo et al. (2005) | [201] |
| 37 | Male | 82 years | 200 mg/day | AH | 12 months | Survived | N/A | Savidou et al. (2006) | [202] |
| 38 | Male | 83 years | 300 mg/day | AH | 7 months | Death | 1.4 months | Savidou et al. (2006) | [202] |
| 39 | Male | 78 years | 300 mg/day | AH | 3 months | Survived | N/A | Savidou et al. (2006) | [202] |
| 40 | Male | 78 years | 150 mg/day | ALF | 2 months | Survived | N/A | Miquel et al. (2007) | [173] |
| 41b | Female | 22 years | 2 mg/day (BCP) | BCS | 7 days | ND | ND | He et al. (2009) | [203][204] |
| 42 | Male | 89 years | 150–300 mg/day | ALF | 3.2 months | Death | 28 days | Kim et al. (2009) | [205] |
| 43 | Male | 71 years | 100–200 mg/day | ALF | 2–3 months | Death | 20 days | Hsu et al. (2011) | [206] |
| 44 | Male | 66 years | 200 mg/day | AH/cirrhosis | 4 months | Survived | N/A | Abenavoli et al. (2013) | [207] |
| 45 | Male | 75 years | 200 mg/day | ALF | 9 months | Survived | N/A | Vodička et al. (2013) | [208] |
| 46 | Male | 87 years | 200 mg/day | ALF | 6 months | Death | 20 days | Kim et al. (2014) | [160] |
| 47 | Male | 80 years | 150 mg/day | AH | 4.0 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 48 | Male | 73 years | 200 mg/day | AH | 2.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 49 | Male | 54 years | 200 mg/day | AIH | 4.0 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 50 | Male | 60 years | 200 mg/day | AH | 1.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 51 | Male | 74 years | 200 mg/day | AH | 5.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 52 | Male | 66 years | 150 mg/day | ALF | 3.2 months | Death | ND | Bessone et al. (2016) | [166] |
| 53 | Male | 77 years | 100 mg/day | AH | 8.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 54 | Male | 72 years | 200 mg/day | AH | 5.0 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 55 | Male | 80 years | 200 mg/day | AH | 1.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 56 | Male | 69 years | 100 mg/day | AH | 4.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 57 | Male | 58 years | 200 mg/day | AH | 10.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 58 | Male | 83 years | 100 mg/day | AH | 2.1 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 59 | Male | 75 years | 200 mg/day | AH | 4.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 60 | Male | 72 years | 100 mg/day | AH | 8.0 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 61 | Male | 72 years | 50 mg/day | AH | 5.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 62 | Male | 66 years | 100 mg/day | AH/CH | 1.2 years | Survived | N/A | Bessone et al. (2016) | [166] |
| 63 | Male | 58 years | 200 mg/day | ALF | 5.0 months | Death | ND | Bessone et al. (2016) | [166] |
| 64 | Male | 75 years | 200 mg/day | ALF | 7.9 months | Death | ND | Bessone et al. (2016) | [166] |
| 65 | Male | 74 years | 150 mg/day | AH | 9.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 66 | Male | 64 years | 100 mg/day | AH | 3.3 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 67 | Male | 64 years | 150 mg/day | AH/CH | 4.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 68 | Male | 64 years | 150 mg/day | AH/cirrhosis | 4.9 months | Survived | N/A | Bessone et al. (2016) | [166] |
| 69 | Male | 61 years | 300 mg/day | ALF | 3 months | Death | 2.6 months | Nour et al. (2017) | [209] |
| Abbreviations: BCP = Birth control pill. RS = Reverse sequential (days 5–25 of cycle). ALF = Acute liver failure (fulminant liver failure). AH = Acute hepatitis. CH = Cholestatic hepatitis. AIH = Autoimmune hepatitis. HCC = Hepatocellular carcinoma. BCS = Budd–Chiari syndrome. ND = No data. N/A = Not applicable. Footnotes: a = Time until death after onset of liver toxicity. b = Probably related to ethinylestradiol rather than to cyproterone acetate.[203] Notes: Many additional cases have been described in spontaneous adverse drug reaction reporting systems of individual countries. These include 19 cases (5 deaths) by late 1988[177] and 96 cases (91 males, 5 females; 33 deaths) by early 1995 in the United Kingdom;[163][164] 32 cases (deaths not given) in Australia by 2004;[210] and 15 cases (no deaths) in Spain by 2006.[211] The cases from Bessone et al. (2016) were reported between 1993 and 2013 and were from Spanish and Latin American drug-induced liver injury databases (17 cases in Argentina, 2 cases in Uruguay, 3 cases in Spain).[166] Worldwide, 153 cases of liver abnormalities were reported to Schering, the manufacturer, between 1982 and 1987.[177] In a large observational study of 2,506 patients, Heinemann et al. (1997) reported 7 cases of benign liver tumors and no cases of serious liver toxicity or HCC.[19] Large observational studies have found no increased risk of liver toxicity or HCC with cyproterone acetate at BCP doses.[19][212][213] A fatal case of ALF in a common chimpanzee has also been reported.[214] Sources: [202][171][196] | |||||||||
Vitamin B12 deficiency
Both low-dose (2 mg/day) and high-dose CPA combined with an estrogen have been associated with vitamin B12 deficiency in women in some small studies.[215][216][7] Vitamin B12 deficiency in turn has been associated with depression, anxiety, irritability, and fatigue due to depletion of central monoamine neurotransmitters.[217][218] For this reason, it has been suggested that low vitamin B12 levels might be involved in the side effect of depression that has sometimes been associated with CPA.[23] Serum vitamin B12 monitoring and supplementation (e.g., 100 to 200 μg/day orally) as necessary may be recommended during CPA therapy.[7][215][216]
Miscellaneous
CPA has been associated rarely with retinal vascular disorder, retinal vein thrombosis, and optic neuritis.[219] A case report of symptomatic epidural lipomatosis in association with CPA therapy has been published.[220] A published case report of lymphocytic pneumonitis in associated with CPA also exists.[4] There is a case report of severe stretch marks in association with CPA therapy.[221]
Dosage dependence
Belisle and Love (1986) directly compared Diane (2 mg/day CPA and 50 μg/day ethinylestradiol) with the combination of 100 mg/day CPA (Androcur) and Diane in 158 women with hirsutism reported no differences in mean incidences of side effects including menometrorrhagia (9.9%), acne (6.5%), decreased libido (9.5%), edema (9.3%), nausea (17.5%), vomiting (4.8%), headache (20.3%), and depression (12.7%).[5] Conversely, the incidences of fatigue and amenorrhea were significantly greater in the Androcur group relative to the Diane group (6.7% vs. 2.5% and 5.4–32.6% vs. 0–4.2%, respectively), the incidence of breast tenderness was significantly lower in the Androcur group than in the Diane group (3.6–26.3% vs. 12.5–46.4%), and the percent gain in body weight relative to baseline was significantly greater in the Androcur group than in the Diane group (1.1% vs. 6.3% at 12 months).[5]
| Side effect | High-dose[lower-alpha 6] (n = 602) (%) | Low-dose[lower-alpha 7] (n = 226) (%) | |
|---|---|---|---|
| Fatigue | 22.0 | 13.0 | |
| Weight gain (>2 kg) | 18.5 | 5.5 | |
| Decreased libido | 10.0 | 6.0 | |
| Breast discomfort | 9.2 | 15.0 | |
| Nausea | 9.0 | 3.9 | |
| Headaches | 7.3 | 10.4 | |
| Depression | 5.1 | 2.0 | |
| Irregular uterine bleeding | 3.5 | 7.2 | |
| Sleep disturbance | 3.5 | – | |
| Thrombophlebitis | 1.0 | 3.0 | |
| Chloasma | 0.9 | – | |
| Constipation | 0.5 | – | |
| Thrombosis | 0.15 | 0.9 | |
| Heavy legs or cramps | – | 4.6 | |
| Sources: See template. | |||
Withdrawal
Adrenal insufficiency
Abrupt withdrawal of CPA can be harmful, and the package insert from Schering AG recommends the daily dose be reduced by no more than 50 mg at intervals of several weeks. The concern is the manner in which CPA affects the adrenal glands. Due to its glucocorticoid activity, high levels of CPA may reduce ACTH, resulting in adrenal insufficiency if discontinued abruptly. In addition, although CPA reduces androgen production in the gonads, it can increase the production of adrenal androgens, in some cases resulting in an overall rise in testosterone levels.[222] Thus, the sudden withdrawal of CPA could result in undesirable androgenic effects. This is a particular concern because androgens, especially DHT, suppress adrenal function, further reducing corticosteroid production.[223]
Suppression of adrenal function and reduced response to adrenocorticotropic hormone (ACTH) have been reported with CPA treatment. As a result, adrenal insufficiency and hence low cortisol and aldosterone levels and ACTH responsiveness can occur upon discontinuation of CPA. Low aldosterone levels may lead to hyponatremia (sodium loss) and hyperkalemia (excess potassium). Patients taking CPA should have their cortisol levels and electrolytes monitored, and if hyperkalemia develops, should reduce the consumption of foods with high potassium content or discontinue the medication.
Notes
- Average dosage; range less than 10 mg/day to >200 mg/day.
- Liver enzymes monitored in 1,685 individuals, including 1,131 males and 554 females.
- Includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancer.
- A subset of the events was combined in a "global index", defined as the earliest occurrence of coronary heart disease events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes.
- Not included in Global Index.
- Reverse sequential regimen: 100 mg/day CPA from day 5 to day 14 of the menstrual cycle and 50 μg/day ethinylestradiol from day 5 to day 25 of the cycle. In women with hirsutism.
- Diane: 2 mg/day CPA, 50 μg/day ethinylestradiol from day 5 to day 25 of the menstrual cycle. In women with hirsutism.
- Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
References
- Bachelot A, Chabbert-Buffet N, Salenave S, Kerlan V, Galand-Portier MB (February 2010). "Anti-androgen treatments". Ann. Endocrinol. (Paris). 71 (1): 19–24. doi:10.1016/j.ando.2009.12.001. PMID 20096826.
- Burton JL (December 1979). "Anti-androgen therapy in dermatology: a review". Clin. Exp. Dermatol. 4 (4): 501–7. doi:10.1111/j.1365-2230.1979.tb01648.x. PMID 394887.
- Hammerstein, J. (1990). "Antiandrogens: Clinical Aspects". Hair and Hair Diseases. pp. 827–886. doi:10.1007/978-3-642-74612-3_35. ISBN 978-3-642-74614-7.
- Rittmaster RS (June 1999). "Antiandrogen treatment of polycystic ovary syndrome". Endocrinol. Metab. Clin. North Am. 28 (2): 409–21. doi:10.1016/S0889-8529(05)70077-3. PMID 10352926.
- Belisle S, Love EJ (December 1986). "Clinical efficacy and safety of cyproterone acetate in severe hirsutism: results of a multicentered Canadian study". Fertil. Steril. 46 (6): 1015–20. doi:10.1016/S0015-0282(16)49873-0. PMID 2946604.
- Diamanti-Kandarakis E (October 1998). "How actual is the treatment with antiandrogen alone in patients with polycystic ovary syndrome?". J. Endocrinol. Invest. 21 (9): 623–9. doi:10.1007/BF03350788. PMID 9856417.
- Diamanti-Kandarakis E (September 1999). "Current aspects of antiandrogen therapy in women". Curr. Pharm. Des. 5 (9): 707–23. PMID 10495361.
- Guay DR (January 2009). "Drug treatment of paraphilic and nonparaphilic sexual disorders". Clin Ther. 31 (1): 1–31. doi:10.1016/j.clinthera.2009.01.009. PMID 19243704.
No quantitative data on these adverse events are available, even in the product prescribing information and data sheets.
- Migliari R, Muscas G, Murru M, Verdacchi T, De Benedetto G, De Angelis M (1999). "Antiandrogens: a summary review of pharmacodynamic properties and tolerability in prostate cancer therapy". Archivio Italiano di Urologia e Andrologia. 71 (5): 293–302. PMID 10673793.
The only advantage of cyproterone acetate on pure antiandrogens seems to be the low incidence of hot flushes; [...] However, hepatotoxicity associated with long term daily doses of 300 mg daily and the unacceptably high incidence of cardiovascular side effects (10%) should restrict its use to patients who are intolerant of pure antiandrogen compound. In contrast to steroidal compound nonsteroidal compounds let sexual potency to be retained, [...]
- Sarah H. Wakelin (1 June 2002). Systemic Drug Treatment in Dermatology: A Handbook. CRC Press. p. 32. ISBN 978-1-84076-013-2.
- Gräf, K.-J.; Brotherton, J.; Neumann, F. (1974). "Clinical Uses of Antiandrogens". Androgens II and Antiandrogens / Androgene II und Antiandrogene. pp. 485–542. doi:10.1007/978-3-642-80859-3_7. ISBN 978-3-642-80861-6.
- Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992.
- Terrence Priestman (26 May 2012). Cancer Chemotherapy in Clinical Practice. Springer Science & Business Media. pp. 97–. ISBN 978-0-85729-727-3.
- Di Lorenzo G, Autorino R, Perdonà S, De Placido S (2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- Hirawat S, Budman DR, Kreis W (June 2003). "The androgen receptor: structure, mutations, and antiandrogens". Cancer Invest. 21 (3): 400–17. doi:10.1081/CNV-120018232. PMID 12901287.
- Schröder FH, Whelan P, de Reijke TM, Kurth KH, Pavone-Macaluso M, Mattelaer J, van Velthoven RF, Debois M, Collette L (April 2004). "Metastatic prostate cancer treated by flutamide versus cyproterone acetate. Final analysis of the "European Organization for Research and Treatment of Cancer" (EORTC) Protocol 30892". Eur. Urol. 45 (4): 457–64. doi:10.1016/j.eururo.2003.11.016. PMID 15041109.
- Shiffman, Melvin A. (2012). "Cellulite: Etiology, Classification, Pathology, and Treatment". Aesthetic Medicine. pp. 265–272. doi:10.1007/978-3-642-20113-4_25. ISBN 978-3-642-20112-7.
- "Mylan-Cyproterone Label" (PDF).
- Heinemann LA, Will-Shahab L, van Kesteren P, Gooren LJ (May 1997). "Safety of cyproterone acetate: report of active surveillance". Pharmacoepidemiol Drug Saf. 6 (3): 169–78. doi:10.1002/(SICI)1099-1557(199705)6:3<169::AID-PDS263>3.0.CO;2-3. PMID 15073785.
- "Androcur Label" (PDF).
- James Barrett (2007). Transsexual and Other Disorders of Gender Identity: A Practical Guide to Management. Radcliffe Publishing. p. 174. ISBN 978-1-85775-719-4.
- Barth JH, Cherry CA, Wojnarowska F, Dawber RP (July 1991). "Cyproterone acetate for severe hirsutism: results of a double-blind dose-ranging study". Clinical Endocrinology. 35 (1): 5–10. doi:10.1111/j.1365-2265.1991.tb03489.x. PMID 1832346.
- Rushton DH (July 2002). "Nutritional factors and hair loss". Clin. Exp. Dermatol. 27 (5): 396–404. doi:10.1046/j.1365-2230.2002.01076.x. PMID 12190640.
- Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, Swanson C, Watson RB, Gardiner RA (September 2002). "Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial" (PDF). BJU Int. 90 (4): 427–32. doi:10.1046/j.1464-410X.2002.02917.x. PMID 12175403.
- Kaisary AV (2005). "Evaluating the use of early hormonal therapy in patients with localised or locally advanced prostate cancer". Prostate Cancer Prostatic Dis. 8 (2): 140–51. doi:10.1038/sj.pcan.4500800. PMID 15852051.
- Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, Swanson CE, Watson RB, Gardiner RA (May 2004). "Quality of life compared during pharmacological treatments and clinical monitoring for non-localized prostate cancer: a randomized controlled trial". BJU Int. 93 (7): 975–9. doi:10.1111/j.1464-410X.2004.04763.x. PMID 15142146.
- Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J (December 2012). "Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens". The Journal of Clinical Endocrinology and Metabolism. 97 (12): 442–8. doi:10.1210/jc.2012-2030. PMID 23055547.
- Gava G, Cerpolini S, Martelli V, Battista G, Seracchioli R, Meriggiola MC (August 2016). "Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness". Clin. Endocrinol. (Oxf). 85 (2): 239–46. doi:10.1111/cen.13050. PMID 26932202.
- Colizzi M, Costa R, Todarello O (January 2014). "Transsexual patients' psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study". Psychoneuroendocrinology. 39: 65–73. doi:10.1016/j.psyneuen.2013.09.029. PMID 24275005.
- Fisher AD, Castellini G, Ristori J, Casale H, Cassioli E, Sensi C, Fanni E, Amato AM, Bettini E, Mosconi M, Dèttore D, Ricca V, Maggi M (November 2016). "Cross-Sex Hormone Treatment and Psychobiological Changes in Transsexual Persons: Two-Year Follow-Up Data" (PDF). J. Clin. Endocrinol. Metab. 101 (11): 4260–4269. doi:10.1210/jc.2016-1276. PMID 27700538.
- Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226.
- Seaman HE, Snowball J, de Vries CS (2006). "Cyproterone Acetate + Ethinyloestradiol and the Risk of Depression". Pharmacoepidemiology and Drug Safety. 15 (S1): S25. doi:10.1002/pds.1295. ISSN 1053-8569. PMID 16986216.
- Husmann, Friedrich (1997). "Clinical Experiences with a Combination of Estradiol Valerate and Cyproterone Acetate for Hormone Replacement". Women's Health and Menopause. Medical Science Symposia Series. 11. pp. 257–261. doi:10.1007/978-94-011-5560-1_38. ISBN 978-94-010-6343-2. ISSN 0928-9550.
- Waken SH, Maibach HI, Archer CB (21 May 2015). Handbook of Systemic Drug Treatment in Dermatology (Second ed.). CRC Press. pp. 34–. ISBN 978-1-4822-2286-9.
- Giltay EJ, Gooren LJ (2009). "Potential side effects of androgen deprivation treatment in sex offenders". J. Am. Acad. Psychiatry Law. 37 (1): 53–8. PMID 19297634.
- Briken P, Hill A, Berner W (August 2003). "Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone-releasing hormone: a systematic review". J Clin Psychiatry. 64 (8): 890–7. doi:10.4088/JCP.v64n0806. PMID 12927003.
- Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". J. Urol. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- Jagielska, Beata; Poniatowska, Grażyna; Tałasiewicz, Konrad; Demkow, Tomasz; Wiechno, Paweł (2017). "Systemic complications in the hormonal treatment of prostate and breast cancer". Nowotwory. Journal of Oncology. 67 (3): 206–214. doi:10.5603/NJO.2017.0034. ISSN 2300-2115.
- Grasswick LJ, Bradford JM (July 2003). "Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports". J. Forensic Sci. 48 (4): 849–55. PMID 12877306.
- Lindsay R (1999). "The lack of effect of progestogen on bone". J Reprod Med. 44 (2 Suppl): 215–20. PMID 11392035.
- Curtis, Kathryn M.; Martins, Summer L. (2006). "Progestogen-only contraception and bone mineral density: a systematic review". Contraception. 73 (5): 470–487. doi:10.1016/j.contraception.2005.12.010. ISSN 0010-7824. PMID 16627031.
- Sarfati, Julie; de Vernejoul, Marie-Christine (2009). "Impact of combined and progestogen-only contraceptives on bone mineral density". Joint Bone Spine. 76 (2): 134–138. doi:10.1016/j.jbspin.2008.09.014. ISSN 1297-319X. PMID 19181558.
- Nelson ER, Wardell SE, McDonnell DP (March 2013). "The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis". Bone. 53 (1): 42–50. doi:10.1016/j.bone.2012.11.011. PMC 3552054. PMID 23168292.
- Spona J, Lunglmayr G (July 1980). Prolaktin-Serumspiegel unter Behandlung des Prostatakarzinoms mit Östradiol-17 beta-undezylat und Cyproteronazetat [Serum Prolactin Levels During Therapy of Prostatic Cancer with Estradiol-17 beta-undecylate and Cyproterone Acetate]. Wien. Klin. Wochenschr. Verhandlungsbericht der Deutschen Gesellschaft für Urologie (in German). 92. pp. 494–7. doi:10.1007/978-3-642-81706-9_120. ISBN 978-3-540-11017-0. ISSN 0043-5325. PMID 6933738.
- Schürmeyer, Th.; Graff, J.; Senge, Th.; Nieschlag, E. (1986). "Effect of oestrogen or cyproterone acetate treatment on adrenocortical function in prostate carcinoma patients". Acta Endocrinologica. 111 (3): 360–367. doi:10.1530/acta.0.1110360. ISSN 0804-4643. PMID 2421511.
- Holub, G.; Lunglmayr, G.; Spona, J. (1981). "Effect of cyproterone/acetate (SH-714) on plasma prolactin in patients with prostatic cancer". Urological Research. 9 (5). doi:10.1007/BF00256895. ISSN 0300-5623. PMID 6458145.
- Rost, A.; Schmidt-Gollwitzer, M.; Hantelmann, W.; Brosig, W. (1981). "Cyproterone acetate, testosterone, LH, FSH, and prolactin levels in plasma after intramuscular application of cyproterone acetate in patients with prostatic cancer". The Prostate. 2 (3): 315–322. doi:10.1002/pros.2990020310. ISSN 0270-4137.
- Jeffcoate, W. J.; Matthews, R. W.; Edwards, C. R. W.; Field, L. H.; Besser, G. M. (1980). "The effect of cyproterone acetate on serum testosterone, LH, FSH, and prolactin in male sexual offenders". Clinical Endocrinology. 13 (2): 189–195. doi:10.1111/j.1365-2265.1980.tb01041.x. ISSN 0300-0664.
- Graf, K. J., Schmidtgollwitzer, M., Koch, U. J., Lorenz, F., & Hammerstein, J. (1978, January). Hyperprolactinemia Induced by Cyproterone Acetate in Human Subjects. In Acta Endocrinologica (Vol. 87, Pp. 96–96).
- Bercovich, E., Cigno, M., & Soli, M. (1983, January). Serum prolactin levels induced by cyproterone acetate therapy in patients with prostatic carcinoma. In Prostate (Vol. 4, No. 4, pp. 425-426).
- Defreyne J, Nota N, Pereira C, Schreiner T, Fisher AD, den Heijer M, T'Sjoen G (October 2017). "Transient Elevated Serum Prolactin in Trans Women Is Caused by Cyproterone Acetate Treatment". LGBT Health. 4 (5): 328–336. doi:10.1089/lgbt.2016.0190. PMID 28880825.
- Fonzo, Domenico; Angeli, Alberto; Sivieri, Roberto; Andriolo, Salvatore; Frajria, Roberto; Ceresa, Franco (1977). "Hyperprolactinemia in Girls with Idiopathic Precocious Puberty Under Prolonged Treatment with Cyproterone Acetate". The Journal of Clinical Endocrinology & Metabolism. 45 (1): 164–168. doi:10.1210/jcem-45-1-164. ISSN 0021-972X.
- Mah, Peak Mann; Webster, Jonathan (2002). "Hyperprolactinemia: Etiology, Diagnosis, and Management". Seminars in Reproductive Medicine. 20 (4): 365–374. doi:10.1055/s-2002-36709. ISSN 1526-8004.
- Gil M, Oliva B, Timoner J, Maciá MA, Bryant V, de Abajo FJ (December 2011). "Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: evidence from a population-based cohort study". Br J Clin Pharmacol. 72 (6): 965–8. doi:10.1111/j.1365-2125.2011.04031.x. PMC 3244644. PMID 21627676.
- Williams, Robert F.; Gianfortoni, Joseph G.; Hodgen, Gary D. (1985). "Hyperprolactinemia Induced by an Estrogen- Progesterone Synergy: Quantitative and Temporal Effects of Estrogen Priming in Monkeys*". The Journal of Clinical Endocrinology & Metabolism. 60 (1): 126–132. doi:10.1210/jcem-60-1-126. ISSN 0021-972X.
- Williams, Robert F.; Barber, Donald L.; Cowan, Bryan D.; Lynch, Almorris; Marut, Edward L.; Hodgen, Gary D. (1981). "Hyperprolactinemia in monkeys: Induction by an estrogen-progesterone synergy". Steroids. 38 (3): 321–331. doi:10.1016/0039-128X(81)90067-2. ISSN 0039-128X.
- Moltz, L.; Römmler, A.; Post, K.; Schwartz, U.; Hammerstein, J. (1980). "Medium dose cyproterone acetate (CPA): Effects on hormone secretion and on spermatogenesis in men". Contraception. 21 (4): 393–413. doi:10.1016/S0010-7824(80)80017-5. ISSN 0010-7824.
- Moltz, L.; Römmler, A.; Schwartz, U.; Post, K.; Hammerstein, J. (1978). "252. Cyproterone acetate (CPA)-a potential male contraceptive: further studies on the interactions with endocrine parameters". Journal of Steroid Biochemistry. 9 (9): 865. doi:10.1016/0022-4731(78)90952-4. ISSN 0022-4731.
- Moltz, L.; Römmler, A.; Schwartz, U.; Hammerstein, J. (1978). "Effects of Cyproterone Acetate (CPA) on Pituitary Gonadotrophin Release and on Androgen Secretion Before and After LH-RH Double Stimulation Tests in Men". International Journal of Andrology. 1 (s2b): 713–719. doi:10.1111/j.1365-2605.1978.tb00518.x. ISSN 0105-6263.
- Meriggiola, M. C.; Costantino, A.; Cerpolini, S.; Bremner, W. J.; Huebler, D.; Morselli-Labate, A. M.; Kirsch, B.; Bertaccini, A.; Pelusi, C.; Pelusi, G. (2003). "Testosterone Undecanoate Maintains Spermatogenic Suppression Induced by Cyproterone Acetate Plus Testosterone Undecanoate in Normal Men". The Journal of Clinical Endocrinology & Metabolism. 88 (12): 5818–5826. doi:10.1210/jc.2003-030574. ISSN 0021-972X.
- Asscheman H, Gooren LJ, Eklund PL (September 1989). "Mortality and morbidity in transsexual patients with cross-gender hormone treatment" (PDF). Metab. Clin. Exp. 38 (9): 869–73. doi:10.1016/0026-0495(89)90233-3. PMID 2528051.
- Asscheman H, Gooren LJ, Assies J, Smits JP, de Slegte R (June 1988). "Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals". Clin. Endocrinol. (Oxf). 28 (6): 583–8. doi:10.1111/j.1365-2265.1988.tb03849.x. PMID 2978262.
- Gooren LJ, Harmsen-Louman W, van Kessel H (February 1985). "Follow-up of prolactin levels in long-term oestrogen-treated male-to-female transsexuals with regard to prolactinoma induction". Clin. Endocrinol. (Oxf). 22 (2): 201–7. doi:10.1111/j.1365-2265.1985.tb01081.x. PMID 3157511.
- Capozzi, Anna; Scambia, Giovanni; Pontecorvi, Alfredo; Lello, Stefano (2015). "Hyperprolactinemia: pathophysiology and therapeutic approach". Gynecological Endocrinology. 31 (7): 506–510. doi:10.3109/09513590.2015.1017810. ISSN 0951-3590.
- Naidoo, U.; Goff, D.C.; Klibanski, A. (2003). "Hyperprolactinemia and bone mineral density: the potential impact of antipsychotic agents". Psychoneuroendocrinology. 28: 97–108. doi:10.1016/S0306-4530(02)00129-4. ISSN 0306-4530. PMID 12650684.
- Rastrelli, Giulia; Corona, Giovanni; Maggi, Mario (2015). "The role of prolactin in andrology: what is new?". Reviews in Endocrine and Metabolic Disorders. 16 (3): 233–248. doi:10.1007/s11154-015-9322-3. ISSN 1389-9155.
- Buvat, J (2003). "Hyperprolactinemia and sexual function in men: a short review". International Journal of Impotence Research. 15 (5): 373–377. doi:10.1038/sj.ijir.3901043. ISSN 0955-9930.
- Krüger, T (2002). "Orgasm-induced prolactin secretion: feedback control of sexual drive?". Neuroscience & Biobehavioral Reviews. 26 (1): 31–44. doi:10.1016/S0149-7634(01)00036-7. ISSN 0149-7634.
- Verhelst, Johan; Abs, Roger (2003). "Hyperprolactinemia". Treatments in Endocrinology. 2 (1): 23–32. doi:10.2165/00024677-200302010-00003. ISSN 1175-6349.
- La Torre D, Falorni A (2007). "harmacological causes of hyperprolactinemia. Therapeutics and Clinical Risk Management". Therapeutics and Clinical Risk Management. 3 (5): 929–951. PMC 2376090. PMID 18473017.
- Frawley, L. Stephen; Porter, Tom E.; Kineman, Rhonda D. (1990). "Effects of Prolactin on Target Cells". Neuroendocrine Perspectives. 8. pp. 39–75. doi:10.1007/978-1-4612-3446-3_2. ISBN 978-1-4612-8014-9. ISSN 0168-0617.
- Bole-Feysot, Christine; Goffin, Vincent; Edery, Marc; Binart, Nadine; Kelly, Paul A. (1998). "Prolactin (PRL) and Its Receptor: Actions, Signal Transduction Pathways and Phenotypes Observed in PRL Receptor Knockout Mice". Endocrine Reviews. 19 (3): 225–268. doi:10.1210/edrv.19.3.0334. ISSN 0163-769X. PMID 9626554.
- Higuchi, Kazumi; Nawata, Hajime; Maki, Toshio; Higashizima, Masayoshi; Kato, Ken-Ichi; Ibayashi, Hiroshi (1984). "Prolactin Has a Direct Effect on Adrenal Androgen Secretion". The Journal of Clinical Endocrinology & Metabolism. 59 (4): 714–718. doi:10.1210/jcem-59-4-714. ISSN 0021-972X.
- Schiebinger, Rick J.; Chrousos, George P.; Culter, Gordon B.; Loriaux, D. Lynn (1986). "The Effect of Serum Prolactin on Plasma Adrenal Androgens and the Production and Metabolic Clearance Rate of Dehydroepiandrosterone Sulfate in Normal and Hyperprolactinemic Subjects". The Journal of Clinical Endocrinology & Metabolism. 62 (1): 202–209. doi:10.1210/jcem-62-1-202. ISSN 0021-972X.
- Langan, Ewan A.; Hinde, Eleanor; Paus, Ralf (2018). "Prolactin as a candidate sebotrop(h)ic hormone?". Experimental Dermatology. 27 (7): 729–736. doi:10.1111/exd.13545. ISSN 0906-6705.
- Pucci E, Petraglia F (December 1997). "Treatment of androgen excess in females: yesterday, today and tomorrow". Gynecol. Endocrinol. 11 (6): 411–33. doi:10.3109/09513599709152569. PMID 9476091.
- Miller JA, Jacobs HS (May 1986). "Treatment of hirsutism and acne with cyproterone acetate". Clin Endocrinol Metab. 15 (2): 373–89. doi:10.1016/S0300-595X(86)80031-7. PMID 2941191.
- Raj R, Korja M, Koroknay-Pál P, Niemelä M (2018). "Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: A report of two cases and a brief literature review". Surg Neurol Int. 9: 109. doi:10.4103/sni.sni_22_18. PMC 5991277. PMID 29930875.
- Nota NM, Wiepjes CM, de Blok C, Gooren LJ, Peerdeman SM, Kreukels B, den Heijer M (July 2018). "The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment". Brain. 141 (7): 2047–2054. doi:10.1093/brain/awy108. PMID 29688280.
- J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2293, 2464, 2479, 6225. ISBN 978-0-323-32195-2.
- Meningeal Neoplasms—Advances in Research and Treatment: 2012 Edition: ScholarlyBrief. ScholarlyEditions. 26 December 2012. pp. 99–. ISBN 978-1-4816-0002-6.
- Dickman A (27 September 2012). Drugs in Palliative Care. OUP Oxford. pp. 137–138. ISBN 978-0-19-966039-1.
- Blankenstein MA, Verheijen FM, Jacobs JM, Donker TH, van Duijnhoven MW, Thijssen JH (2000). "Occurrence, regulation, and significance of progesterone receptors in human meningioma". Steroids. 65 (10–11): 795–800. doi:10.1016/S0039-128X(00)00193-8. PMID 11108890.
- Shlomo Melmed (9 December 2010). The Pituitary. Academic Press. pp. 227–. ISBN 978-0-12-380927-8.
- Knobil and Neill's Physiology of Reproduction. Academic Press. 12 December 2005. pp. 1557–. ISBN 978-0-08-053527-2.
- Marc A. Fritz; Leon Speroff (2011). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 1091–. ISBN 978-0-7817-7968-5.
- Mancini I, Rotilio A, Coati I, Seracchioli R, Martelli V, Meriggiola MC (June 2018). "Presentation of a meningioma in a transwoman after nine years of cyproterone acetate and estradiol intake: case report and literature review". Gynecol. Endocrinol. 34 (6): 456–459. doi:10.1080/09513590.2017.1395839. PMID 29105524.
- Schmutz JL (May 2018). "Méningiomes et acétate de cyprotérone: mise au point" [Cyproterone acetate and meningioma: The latest findings]. Ann Dermatol Venereol (in French). 145 (5): 390–391. doi:10.1016/j.annder.2018.04.001. PMID 29703641.
- Ter Wengel PV, Martin E, Gooren L, Den Heijer M, Peerdeman SM (December 2016). "Meningiomas in three male-to-female transgender subjects using oestrogens/progestogens and review of the literature". Andrologia. 48 (10): 1130–1137. doi:10.1111/and.12550. PMID 26888610.
- Gruber CJ, Huber JC (December 2003). "Differential effects of progestins on the brain". Maturitas. 46 Suppl 1: S71–5. doi:10.1016/j.maturitas.2003.09.021. PMID 14670648.
- Hensiek AE, Kellerman AJ, Hill JT (August 2000). "Spontaneous regression of a solitary cerebral metastases in renal carcinoma followed by meningioma development under medroxyprogesterone acetate therapy". Br J Neurosurg. 14 (4): 354–6. doi:10.1080/026886900417388. PMID 11045205.
- Gooren LJ, Assies J, Asscheman H, de Slegte R, van Kessel H (February 1988). "Estrogen-induced prolactinoma in a man". J. Clin. Endocrinol. Metab. 66 (2): 444–6. doi:10.1210/jcem-66-2-444. PMID 3339116.
- Serri O, Noiseux D, Robert F, Hardy J (September 1996). "Lactotroph hyperplasia in an estrogen treated male-to-female transsexual patient". J. Clin. Endocrinol. Metab. 81 (9): 3177–9. doi:10.1210/jcem.81.9.8784065. PMID 8784065.
- García-Malpartida K, Martín-Gorgojo A, Rocha M, Gómez-Balaguer M, Hernández-Mijares A (August 2010). "Prolactinoma induced by estrogen and cyproterone acetate in a male-to-female transsexual". Fertil. Steril. 94 (3): 1097.e13–5. doi:10.1016/j.fertnstert.2010.01.076. PMID 20227072.
- Bunck MC, Debono M, Giltay EJ, Verheijen AT, Diamant M, Gooren LJ (2009). "Autonomous prolactin secretion in two male-to-female transgender patients using conventional oestrogen dosages". BMJ Case Rep. 2009: bcr0220091589. doi:10.1136/bcr.02.2009.1589. PMC 3029513. PMID 21829422.
- Cunha FS, Domenice S, Câmara VL, Sircili MH, Gooren LJ, Mendonça BB, Costa EM (August 2015). "Diagnosis of prolactinoma in two male-to-female transsexual subjects following high-dose cross-sex hormone therapy". Andrologia. 47 (6): 680–4. doi:10.1111/and.12317. PMID 25059808.
- Gazzeri R, Galarza M, Gazzeri G (December 2007). "Growth of a meningioma in a transsexual patient after estrogen-progestin therapy". N. Engl. J. Med. 357 (23): 2411–2. doi:10.1056/NEJMc071938. PMID 18057351.
- Deipolyi AR, Han SJ, Parsa AT (October 2010). "Development of a symptomatic intracranial meningioma in a male-to-female transsexual after initiation of hormone therapy". J Clin Neurosci. 17 (10): 1324–6. doi:10.1016/j.jocn.2010.01.036. PMID 20594855.
- Cebula H, Pham TQ, Boyer P, Froelich S (November 2010). "Regression of meningiomas after discontinuation of cyproterone acetate in a transsexual patient". Acta Neurochir (Wien). 152 (11): 1955–6. doi:10.1007/s00701-010-0787-2. PMID 20811919.
- Bergoglio MT, Gómez-Balaguer M, Almonacid Folch E, Hurtado Murillo F, Hernández-Mijares A (May 2013). "Symptomatic meningioma induced by cross-sex hormone treatment in a male-to-female transsexual". Endocrinol Nutr. 60 (5): 264–7. doi:10.1016/j.endonu.2012.07.004. PMID 23022362.
- Mueller A, Gooren L (September 2008). "Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones". Eur. J. Endocrinol. 159 (3): 197–202. doi:10.1530/EJE-08-0289. PMID 18567667.
- Cea-Soriano L, Blenk T, Wallander MA, Rodríguez LA (April 2012). "Hormonal therapies and meningioma: is there a link?". Cancer Epidemiol. 36 (2): 198–205. doi:10.1016/j.canep.2011.08.003. PMID 21943794.
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (November 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline". J. Clin. Endocrinol. Metab. 102 (11): 3869–3903. doi:10.1210/jc.2017-01658. PMID 28945902.
- McFarlane T, Zajac JD, Cheung AS (December 2018). "Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals-A systematic review". Clin. Endocrinol. (Oxf). 89 (6): 700–711. doi:10.1111/cen.13835. PMID 30107028.
- Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 544–. ISBN 978-3-642-60107-1.
- Kovacs K, Stefaneanu L, Ezzat S, Smyth HS (May 1994). "Prolactin-producing pituitary adenoma in a male-to-female transsexual patient with protracted estrogen administration. A morphologic study". Arch. Pathol. Lab. Med. 118 (5): 562–5. PMID 8192565.
- Giraldi, Laura; Hansen, Jørgen Vinsløv; Wohlfahrt, Jan; Melbye, Mads; Fugleholm, Kåre; Munch, Tina Nørgaard (2019). "Male hormone-interfering drugs and meningioma development". Neuro-Oncology Advances. doi:10.1093/noajnl/vdz046. ISSN 2632-2498.
- van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ (September 1997). "Mortality and morbidity in transsexual subjects treated with cross-sex hormones". Clin. Endocrinol. (Oxf). 47 (3): 337–42. doi:10.1046/j.1365-2265.1997.2601068.x. PMID 9373456.
- Futterweit W (April 1998). "Endocrine therapy of transsexualism and potential complications of long-term treatment". Arch Sex Behav. 27 (2): 209–26. doi:10.1023/A:1018638715498. PMID 9562902.
- Gonçalves AM, Page P, Domigo V, Méder JF, Oppenheim C (September 2010). "Abrupt regression of a meningioma after discontinuation of cyproterone treatment". AJNR Am J Neuroradiol. 31 (8): 1504–5. doi:10.3174/ajnr.A1978. PMID 20053802.
- Knight, Ema J.; McDonald, Matthew J. (2013). "Recurrence and Progression of Meningioma in Male-to-Female Transgender Individuals During Exogenous Hormone Use". International Journal of Transgenderism. 14 (1): 18–23. doi:10.1080/15532739.2012.725563. ISSN 1553-2739.
- Razavi, Hamid Borghei (2014). "Meningioma: The Unusual Growth in a Transsexual Patient after Estrogen-Progesterone Therapy". SOJ Neurology. 1 (2). doi:10.15226/2374-6858/1/2/00109. ISSN 2374-6858.
- Bernat AL, Oyama K, Hamdi S, Mandonnet E, Vexiau D, Pocard M, George B, Froelich S (October 2015). "Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: a case series of 12 patients". Acta Neurochir (Wien). 157 (10): 1741–6. doi:10.1007/s00701-015-2532-3. PMID 26264069.
- Botella C, Coll G, Lemaire JJ, Irthum B (October 2015). "Méningiomes intracrâniens et utilisation prolongée d'acétate de cyprotérone à dose conventionnelle chez la femme : à propos de deux cas de régression tumorale après arrêt du traitement" [Intra cranial meningiomas and long term use of cyproterone acetate with a conventional dose in women. A report of two cases of tumor decrease after treatment withdrawal]. Neurochirurgie (in French). 61 (5): 339–42. doi:10.1016/j.neuchi.2015.05.002. PMID 26249273.
- Sys C, Kestelyn P (2015). "Unilateral proptosis and blindness caused by meningioma in a patient treated with cyproterone acetate". GMS Ophthalmol Cases. 5: Doc05. doi:10.3205/oc000027. PMC 5015623. PMID 27625949.
- Kalamarides M, Peyre M (May 2017). "Dramatic Shrinkage with Reduced Vascularization of Large Meningiomas After Cessation of Progestin Treatment". World Neurosurg. 101: 814.e7–814.e10. doi:10.1016/j.wneu.2017.03.013. PMID 28300711.
- Keilani C, Abada S (May 2017). "An uncommon case of symptomatic multiple meningiomas with bilateral compressive optic neuropathy rapidly induced under cyproterone acetate treatment". Curr Drug Saf. doi:10.2174/1574886312666170523154548. PMID 28545357.
- Boer, Mirra; Moernaut, Loes; Van Calenbergh, Frank; Lapauw, Bruno; T'Sjoen, Guy (2018). "Variation of meningioma in response to cyproterone acetate in a trans woman". International Journal of Transgenderism. 19 (1): 92–94. doi:10.1080/15532739.2017.1413615. ISSN 1553-2739.
- Froelich S, Dali-Youcef N, Boyer P, Kehrli P, Maitrot D, Auwerx J, Schlienger JL (2008). "Does cyproterone acetate promote multiple meningiomas?". Endocrine Abstracts. 16: P158. ISSN 1470-3947.
- Vasilakis-Scaramozza C, Jick H (October 2001). "Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives". Lancet. 358 (9291): 1427–9. doi:10.1016/S0140-6736(01)06522-9. PMID 11705493.
- Spitzer WO (December 2003). "Cyproterone acetate with ethinylestradiol as a risk factor for venous thromboembolism: an epidemiological evaluation". J Obstet Gynaecol Can. 25 (12): 1011–8. doi:10.1016/S1701-2163(16)30342-5. PMID 14663535.
- Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E (October 2011). "Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9". BMJ. 343: d6423. doi:10.1136/bmj.d6423. PMC 3202015. PMID 22027398.
- Kromm J, Jeerakathil T (June 2014). "Cyproterone acetate-ethinyl estradiol use in a 23-year-old woman with stroke". CMAJ. 186 (9): 690–3. doi:10.1503/cmaj.130579. PMC 4049993. PMID 24491473.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Wiegratz I, Kuhl H (September 2006). "Metabolic and clinical effects of progestogens". Eur J Contracept Reprod Health Care. 11 (3): 153–61. doi:10.1080/13625180600772741. PMID 17056444.
- Asscheman H, T'Sjoen G, Lemaire A, Mas M, Meriggiola MC, Mueller A, Kuhn A, Dhejne C, Morel-Journel N, Gooren LJ (September 2014). "Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review". Andrologia. 46 (7): 791–5. doi:10.1111/and.12150. PMID 23944849.
- Beyer-Westendorf J, Werth S, Halbritter K, Weiss N (April 2010). "Cancer in males and risk of venous thromboembolism". Thromb. Res. 125 Suppl 2: S155–9. doi:10.1016/S0049-3848(10)70035-9. PMID 20433997.
- Guay DR (December 2008). "Inappropriate sexual behaviors in cognitively impaired older individuals". Am J Geriatr Pharmacother. 6 (5): 269–88. doi:10.1016/j.amjopharm.2008.12.004. PMID 19161930.
- Seaman HE, Langley SE, Farmer RD, de Vries CS (June 2007). "Venous thromboembolism and cyproterone acetate in men with prostate cancer: a study using the General Practice Research Database". BJU Int. 99 (6): 1398–403. doi:10.1111/j.1464-410X.2007.06859.x. PMID 17537215.
- Van Hemelrijck M, Adolfsson J, Garmo H, Bill-Axelson A, Bratt O, Ingelsson E, Lambe M, Stattin P, Holmberg L (May 2010). "Risk of thromboembolic diseases in men with prostate cancer: results from the population-based PCBaSe Sweden". Lancet Oncol. 11 (5): 450–8. doi:10.1016/S1470-2045(10)70038-3. PMC 2861771. PMID 20395174.
- Namer M (October 1988). "Clinical applications of antiandrogens". J. Steroid Biochem. 31 (4B): 719–29. doi:10.1016/0022-4731(88)90023-4. PMID 2462132.
- Jacobi, GR; Tunn, UW; Senge, TH (1 December 1982). "Clinical experience with cyproterone acetate for palliation of inoperable prostate cancer". In Jacobi, Günther H; Hohenfellner, Rudolf (eds.). Prostate Cancer. Williams & Wilkins. pp. 305–319. ISBN 978-0-683-04354-9.
- Schröder, Fritz H.; Radlmaier, Albert (2009). "Steroidal Antiandrogens". In V. Craig Jordan; Barrington J. A. Furr (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
- Schröder FH (1998). "Antiandrogens as monotherapy for prostate cancer". European Urology. 34 Suppl 3: 12–7. doi:10.1159/000052291. PMID 9854190.
- Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H (October 2018). "Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy". VASA. 47 (6): 441–450. doi:10.1024/0301-1526/a000726. PMID 30008249.
- DeLoughery TG (June 2011). "Estrogen and thrombosis: controversies and common sense". Rev Endocr Metab Disord. 12 (2): 77–84. doi:10.1007/s11154-011-9178-0. PMID 21559819.
- Van Hylckama Vlieg, A.; Middeldorp, S. (2011). "Hormone therapies and venous thromboembolism: where are we now?". Journal of Thrombosis and Haemostasis. 9 (2): 257–266. doi:10.1111/j.1538-7836.2010.04148.x. ISSN 1538-7933. PMID 21114755.
- Benagiano, G.; Primiero, F.M. (1983). "Long Acting Contraceptives Present Status". Drugs. 25 (6): 570–609. doi:10.2165/00003495-198325060-00003. ISSN 0012-6667. PMID 6223801.
- Mantha, S.; Karp, R.; Raghavan, V.; Terrin, N.; Bauer, K. A.; Zwicker, J. I. (2012). "Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis". BMJ. 345 (aug07 2): e4944. doi:10.1136/bmj.e4944. ISSN 1756-1833.
- Singh, Shankar; Gauthier, Sylvain; Labrie, Fernand (2000). "Androgen Receptor Antagonists (Antiandrogens) Structure-Activity Relationships". Current Medicinal Chemistry. 7 (2): 211–247. doi:10.2174/0929867003375371. ISSN 0929-8673. PMID 10637363.
When compared to flutamide, [cyproterone acetate] has significant intrinsic androgenic and estrogenic activities. [...] The effects of flutamide and the steroidal derivatives, cyproterone acetate, chlormadinone acetate, megestrol acetate and medroxyprogesterone acetate were compared in vivo in female nude mice bearing androgen-sensitive Shionogi tumors. All steroidal compounds stimulated tumor growth while flutamide had no stimulatory effect [51]. Thus, CPA due to its intrinsic properties stimulates androgen-sensitive parameters and cancer growth. Cyproterone acetate added to castration has never been shown in any controlled study to prolong disease-free survival or overall survival in prostate cancer when compared with castration alone [152-155].
- Newling DW (March 1997). "The palliative therapy of advanced prostate cancer, with particular reference to the results of recent European clinical trials". Br J Urol. 79 Suppl 1: 72–81. doi:10.1111/j.1464-410X.1997.tb00805.x. PMID 9088277.
- Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clin Pharmacokinet. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 150–152. ISBN 978-0-08-093292-7.
- Müller E (18 September 2003). Peptides and Non Peptides of Oncologic and Neuroendocrine Relevance: From Basic to Clinical Research. Springer Science & Business Media. pp. 171–. ISBN 978-88-470-0295-1. Archived from the original on 8 September 2017.
[CPA] induces relevant effects on the coagulative system. A recent meta-analysis relating to total androgenic blockade has shown that cyproterone acetate when combined with castration reduces the long-term efficacy of androgen-suppressive treatments. In fact, it causes an increase in treatment-related mortality, mainly due to cardiovascular complications (No authors, 2000).
- Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- Chang C (22 March 2005). Prostate Cancer: Basic Mechanisms and Therapeutic Approaches. World Scientific. pp. 10–11. ISBN 978-981-4481-61-8. Archived from the original on 8 September 2017.
- Gillatt D (August 2006). "Antiandrogen treatments in locally advanced prostate cancer: are they all the same?". J. Cancer Res. Clin. Oncol. 132 Suppl 1: S17–26. doi:10.1007/s00432-006-0133-5. PMID 16845534.
- Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116.
- Stanczyk FZ, Bhavnani BR (July 2014). "Use of medroxyprogesterone acetate for hormone therapy in postmenopausal women: is it safe?". J. Steroid Biochem. Mol. Biol. 142: 30–8. doi:10.1016/j.jsbmb.2013.11.011. PMID 24291402.
- Sturdee DW (2013). "Are progestins really necessary as part of a combined HRT regimen?". Climacteric. 16 Suppl 1: 79–84. doi:10.3109/13697137.2013.803311. PMID 23651281.
- Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- Neumann F, Kalmus J (1991). "Cyproterone acetate in the treatment of sexual disorders: pharmacological base and clinical experience". Exp. Clin. Endocrinol. 98 (2): 71–80. doi:10.1055/s-0029-1211103. PMID 1838080.
- Ahmadi H, Daneshmand S (2014). "Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes". Patient Relat Outcome Meas. 5: 63–70. doi:10.2147/PROM.S52788. PMC 4094624. PMID 25045284.
- Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- Dalesio O, van Tinteren H, Clarke M, Peto R, Schroder F, Dechering I, Evans V, Godwin J, Blumenstein B, Crawford E, Denis L, Hall R, Hill C, Iversen P, Shipley W, Soloway M, Sylvester R (2000). "Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials". The Lancet. 355 (9214): 1491–1498. doi:10.1016/S0140-6736(00)02163-2. ISSN 0140-6736.
- Chodak G, Gomella L, Phung de H (September 2007). "Combined androgen blockade in advanced prostate cancer: looking back to move forward". Clin Genitourin Cancer. 5 (6): 371–8. doi:10.3816/CGC.2007.n.019. PMID 17956709.
- DeLeve, Laurie D. (2013). "Cancer Chemotherapy". Drug-Induced Liver Disease. pp. 541–567. doi:10.1016/B978-0-12-387817-5.00030-3. ISBN 9780123878175.
- Kim JH, Yoo BW, Yang WJ (May 2014). "Hepatic failure induced by cyproterone acetate: A case report and literature review". Can Urol Assoc J. 8 (5–6): E458–61. doi:10.5489/cuaj.1753. PMC 4081269. PMID 25024808.
- Savidou I, Deutsch M, Soultati AS, Koudouras D, Kafiri G, Dourakis SP (December 2006). "Hepatotoxicity induced by cyproterone acetate: a report of three cases". World Journal of Gastroenterology. 12 (46): 7551–5. doi:10.3748/wjg.v12.i46.7551. PMC 4087608. PMID 17167851.
- Hinkel A, Berges RR, Pannek J, Schulze H, Senge T (1996). "Cyproterone acetate in the treatment of advanced prostatic cancer: retrospective analysis of liver toxicity in the long-term follow-up of 89 patients". Eur. Urol. 30 (4): 464–70. doi:10.1159/000474216. PMID 8977068.
- Rabe T, Feldmann K, Heinemann L, Runnebaum B (January 1996). "Cyproterone acetate: is it hepato- or genotoxic?". Drug Saf. 14 (1): 25–38. doi:10.2165/00002018-199614010-00004. PMID 8713486.
Recently, a publication of the Medicines Control Agency (MCA)/Committee on Safety of Medicines (CSM)[5] drew attention to spontaneous reports of serious and dose-related hepatic toxicity after the prolonged use of CPA. [...] 96 hepatotoxic events (33 fatal) have been observed, of which 91 were in males and 5 in females. The hepatic reactions included hepatitis, cholestatic jaundice and hepatic failure. The majority of patients were treated with high dosages of CPA (300 mg/day) for cancer of the prostate.
- "Hepatic reactions with cyproterone acetate (Cyprostat, Androcur)". Current Problems in Pharmacovigilance (21): 1. February 1995.
- Gava, Giulia; Seracchioli, Renato; Meriggiola, Maria Cristina (2017). "Therapy with Antiandrogens in Gender Dysphoric Natal Males". Endocrinology of the Testis and Male Reproduction. Endocrinology. pp. 1199–1209. doi:10.1007/978-3-319-44441-3_42. ISBN 978-3-319-44440-6. ISSN 2510-1927.
- Bessone F, Lucena MI, Roma MG, Stephens C, Medina-Cáliz I, Frider B, Tsariktsian G, Hernández N, Bruguera M, Gualano G, Fassio E, Montero J, Reggiardo MV, Ferretti S, Colombato L, Tanno F, Ferrer J, Zeno L, Tanno H, Andrade RJ (February 2016). "Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases". Liver Int. 36 (2): 302–10. doi:10.1111/liv.12899. PMID 26104271.
- Marius Duker (23 May 2016). Incapacitation: Trends and New Perspectives. Routledge. pp. 139–. ISBN 978-1-317-11767-4.
- Berlex Canada, Inc. (2003-02-10). "Cyproterone Acetate Tablets and Injections Product Monographs (revised version)" (PDF). Archived from the original (PDF) on 2006-09-24.
- Adverse Drug Reactions Advisory Committee (February 2004). "Australian Adverse Drug Reactions Bulletin, Volume 23, Number 1".
- Lin AD, Chen KK, Lin AT, Chang YH, Wu HH, Kuo JY, Huang WJ, Hsu YS, Chung HJ, Chang LS (December 2003). "Antiandrogen-associated hepatotoxicity in the management of advanced prostate cancer". J Chin Med Assoc. 66 (12): 735–40. PMID 15015823.
- Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urol. Int. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569.
- Chitturi, Shivakumar; Farrell, Geoffrey C (2013). "Adverse Effects of Hormones and Hormone Antagonists on the Liver". Drug-Induced Liver Disease. pp. 605–619. doi:10.1016/B978-0-12-387817-5.00033-9. ISBN 9780123878175.
- Miquel M, Soler A, Vaqué A, Ojanguren I, Costa J, Planas R (October 2007). "Suspected cross-hepatotoxicity of flutamide and cyproterone acetate". Liver Int. 27 (8): 1144–7. doi:10.1111/j.1478-3231.2007.01549.x. PMID 17845544.
- Manolakopoulos S, Bethanis S, Armonis A, Economou M, Avgerinos A, Tzourmakliotis D (March 2004). "Toxic hepatitis after sequential administration of flutamide and cyproterone acetate". Dig. Dis. Sci. 49 (3): 462–5. doi:10.1023/B:DDAS.0000020504.41500.9c. PMID 15139499.
- Meijers WH, Willemse PH, Sleijfer DT, Mulder NH, Grond J (September 1986). "Hepatocellular damage by cyproterone acetate". Eur J Cancer Clin Oncol. 22 (9): 1121–2. doi:10.1016/0277-5379(86)90017-9. PMID 2946585.
- Lévesque H, Trivalle C, Manchon ND, Vinel JP, Moore N, Hémet J, Courtois H, Bercoff E, Bourreille J (January 1989). "Fulminant hepatitis due to cyproterone acetate". Lancet. 1 (8631): 215–6. doi:10.1016/S0140-6736(89)91225-7. PMID 2563116.
- Blake JC, Sawyerr AM, Dooley JS, Scheuer PJ, McIntyre N (May 1990). "Severe hepatitis caused by cyproterone acetate". Gut. 31 (5): 556–7. doi:10.1136/gut.31.5.556. PMC 1378574. PMID 2140997.
Although few case reports have been published, the Committee on Safety of Medicines had received 19 reports in the UK of adverse hepatic reactions associated with cyproterone acetate by November 1988 (personal communication, Committee on Safety of Medicines). Five of these cases were fatal.
- Dore B, Orget J, Irani J, Aubert J (1990). "Hépatite après traitement par acétate de cyprotérone. A propos d'un cas" [Hepatitis after treatment with cyproterone acetate. Apropos of a case]. J Urol (Paris) (in French). 96 (3): 169–71. ISSN 0248-0018. PMID 2145371.
- Antoni M, Bourlière M, Toullec J, Maillot A, Botta-Fridlund D, Gauthier A (1991). "Hepatite sub-fulminante d'evolution mortelle a l'acetate de cyproterone" [Fatal subfulminant hepatitis caused by cyproterone acetate]. Gastroenterol. Clin. Biol. (in French). 15 (10): 772–3. ISSN 0399-8320. PMID 1840042.
- Ohri SK, Gaer JA, Keane PF (February 1991). "Hepatocellular carcinoma and treatment with cyproterone acetate". Br J Urol. 67 (2): 213. doi:10.1111/j.1464-410X.1991.tb15113.x. PMID 1848454.
- Parys BT, Hamid S, Thomson RG (March 1991). "Severe hepatocellular dysfunction following cyproterone acetate therapy". Br J Urol. 67 (3): 312–3. doi:10.1111/j.1464-410X.1991.tb15142.x. PMID 1827039.
- Drakos PE, Gez E, Catane R (1992). "Hepatitis due to cyproterone acetate". Eur. J. Cancer. 28A (11): 1931–2. doi:10.1016/0959-8049(92)90041-Y. PMID 1389539.
- Hassler P, Duchêne R (1992). "Hépatotoxicité de l'acétate de cyprotérone" [Hepatotoxicity of cyproterone acetate]. Rev Med Interne (in French). 13 (3): 245. doi:10.1016/S0248-8663(05)81339-6. PMID 1410910.
- Roila F, Crinò L, Carloni G, Natalini G (September 1993). "Cyproterone acetate: hepatotoxicity and prostatic cancer treatment". Ann. Oncol. 4 (8): 701. doi:10.1093/oxfordjournals.annonc.a058631. PMID 8241005.
- Bressollette L, Dubois A, Carlhant D, Morand C, Mottier D, Riche C (1994). "Hépatite mortelle a l'acétate de cyprotérone" [Fatal hepatitis caused by cyproterone acetate]. Therapie (in French). 49 (2): 153. PMID 7817350.
- Hirsch D, Kovatz S, Bernheim J, Shenkman L (March 1994). "Fatal fulminant hepatitis from cyproterone acetate". Isr. J. Med. Sci. 30 (3): 238–40. PMID 8181926.
- Kattan J, Spatz A, Culine S, Terrier-Lacombe MJ, Elias D, Droz JP (October 1994). "Hepatocellular carcinoma during hormonotherapy for prostatic cancer". Am. J. Clin. Oncol. 17 (5): 390–2. doi:10.1097/00000421-199410000-00006. PMID 8092108.
- Watanabe S, Yamasaki S, Tanae A, Hibi I, Honna T (December 1994). "Three cases of hepatocellular carcinoma among cyproterone users. Ad hoc Committee on Androcur Users". Lancet. 344 (8936): 1567–8. doi:10.1016/S0140-6736(94)90373-5. PMID 7983963.
- Watanabe S, Cui Y, Tanae A, Tanaka T, Fujimoto M, Matsuo Y, Tachibana K, Yamasaki S (September 1997). "Follow-up study of children with precocious puberty treated with cyproterone acetate. Ad hoc Committee for CPA". J Epidemiol. 7 (3): 173–8. doi:10.2188/jea.7.173. PMID 9337516.
- Pinganaud G, Chaslerie A, Bourdel Marchasson I, Decamps A, Manciet G, Emeriau JP (June 1995). "Cyproterone-induced hepatotoxicity". Ann Pharmacother. 29 (6): 634. doi:10.1177/106002809502900619. PMID 7663042.
- Rüdiger T, Beckmann J, Queisser W (February 1995). "Hepatocellular carcinoma after treatment with cyproterone acetate combined with ethinyloestradiol". Lancet. 345 (8947): 452–3. doi:10.1016/S0140-6736(95)90434-4. PMID 7853970.
- Castellani P, Bernardini D, Renou C, Zamora C, Portal I, Gauthier A, Botta-Fridlund D (1996). "Hépatite subfulminante mortelle à l'acétate de cyprotérone. Un nouveau cas" [Fatal sub-fulminant hepatitis caused by cyproterone acetate. A new case]. Gastroenterol. Clin. Biol. (in French). 20 (10): 915–6. ISSN 0399-8320. PMID 8991155.
- Murphy BJ, Collins BJ (October 1996). "Severe hepatitis and liver failure induced by cyproterone acetate". Aust N Z J Med. 26 (5): 724. doi:10.1111/j.1445-5994.1996.tb02956.x. PMID 8958378.
- Ruiz-Rebollo ML, Polo F, Palenzuela R, Moretó M (1997). "Hepatitis aguda severa por ciproterona" [Severe acute hepatitis due to cyproterone]. Gastroenterol Hepatol (in Spanish; Castilian). 20 (7): 385. ISSN 0210-5705. PMID 9377242.CS1 maint: unrecognized language (link)
- Lombardi A, Ferrazza P, Castaldi F, Covotta L, Tesoriere A, Urbano V, Midiri G (April 1998). "[Acute hepatic necrosis in a patient treated with cyproterone acetate]". G Chir (in Italian). 19 (4): 161–3. PMID 9628065.
- Friedman G, Lamoureux E, Sherker AH (July 1999). "Fatal fulminant hepatic failure due to cyproterone acetate". Dig. Dis. Sci. 44 (7): 1362–3. doi:10.1023/A:1026639432428. PMID 10489919.
- Garty BZ, Dinari G, Gellvan A, Kauli R (May 1999). "Cirrhosis in a child with hypothalamic syndrome and central precocious puberty treated with cyproterone acetate". Eur. J. Pediatr. 158 (5): 367–70. doi:10.1007/s004310051093. PMID 10333116.
- Manfredi S, Lenfant L, Gresset AC, Bonnotte B, Lorcerie B, Chauffert B (November 2000). "Carcinome hépatocellulaire sur foie sain potentiellement imputable à la prise au long cours d'acétate de cyprotérone" [Hepatocellular carcinoma in a healthy liver possibly due to long-term use of cyproterone acetate]. Presse Med (in French). 29 (36): 1983. ISSN 0755-4982. PMID 11149080.
- Giordano N, Nardi P, Santacroce C, Geraci S, Gennari C (September 2001). "Acute hepatitis induced by cyproterone acetate". Ann Pharmacother. 35 (9): 1053–5. doi:10.1177/106002800103500902. PMID 11573856.
- Kacar S, Akdogan M, Koşar Y, Parlak E, Sasmaz N, Oguz P, Aydog G (July 2002). "Estrogen and cyproterone acetate combination-induced autoimmune hepatitis". J. Clin. Gastroenterol. 35 (1): 98–100. doi:10.1097/00004836-200207000-00023. PMID 12080237.
- Famularo G, Minisola G, Grieco A, Miele L (September 2005). "Fulminant liver failure caused by cyproterone". Dig Liver Dis. 37 (9): 718–9. doi:10.1016/j.dld.2005.04.018. PMID 15936995.
- Savidou I, Deutsch M, Soultati AS, Koudouras D, Kafiri G, Dourakis SP (December 2006). "Hepatotoxicity induced by cyproterone acetate: a report of three cases". World J. Gastroenterol. 12 (46): 7551–5. doi:10.3748/wjg.v12.i46.7551. PMC 4087608. PMID 17167851.
- He JC, Xu P, Peng LB (December 2009). "[A case of Budd-Chiari syndrome induced by ethinylestradiol and cyproterone acetate]". Zhonghua Gan Zang Bing Za Zhi (in Chinese). 17 (12): 954. doi:10.3760/cma.j.issn.1007-3418.2009.12.020. ISSN 1007-3418. PMID 20038345.
- "Ethinylestradiol/cyproterone. Budd-Chiari syndrome: case report". Reactions Weekly (1340): 20. February 2011. doi:10.2165/00128415-201113400-00066. ISSN 0114-9954.
- Kim BH, Kim DJ, Sohn KM, Yang HN, Choi MJ, Lee CW, Choi KC (2009). "잠복간경변 환자에서 cyproterone acetate에 의해 발생한 전격간부전 1예" [A case of fulminant hepatic failure due to cyproterone acetate in a patient with cryptogenic liver cirrhosis]. Korean J Med. 77 (Suppl 1): S31–5. ISSN 1738-9364.
- Hsu YC, Tai DI (2011). "Unusually high alanine aminotransferase to aspartate aminotransferase ratio in a patient with cyproterone-induced icteric hepatitis". Chang Gung Med J. 34 (6 Suppl): 34–8. PMID 22490456.
- Abenavoli L, Milic N, Beaugrand M (2013). "Severe hepatitis induced by cyproterone acetate: role of corticosteroids. A case report". Ann Hepatol. 12 (1): 152–5. PMID 23293208.
- Vodička M, Sálek T, Röderová E, Cerný D (2013). "Hepatotoxicita po cyproteron acetátu v léčbě karcinomu prostaty – kazuistika" [Hepatotoxicity induced by cyproterone acetate in the prostate carcinoma treatment - a case report]. Klin Onkol (in Czech). 26 (1): 47–8. PMID 23528173.
- Nour E, Mehdi K, Hanene J, Hammami A, Ben Slama A, Ali J (December 2017). "Fatal acute liver failure induced by cyproterone acetate: A new case". Presse Med. 46 (12 Pt 1): 1231–1232. doi:10.1016/j.lpm.2017.09.003. PMID 29129412.
- "High dose cyproterone and hepatotoxicity - Australian Adverse Drug Reactions Bulletin, Vol 23, No 1". Australian Adverse Drug Reactions Bulletin. 23 (1): 3. 2004. ISSN 1325-8540.
High dose cyproterone (50mg, 100mg; Androcur, Androcur-100) is used predominantly for advanced prostate carcinoma. For the year ending June 2003, 59,000 prescriptions were dispensed for the 50mg or 100mg tablets and 97% of patients prescribed these tablets were male. [...] Over the years, ADRAC has received 105 reports implicating high-dose cyproterone. The most common adverse reactions are related to the liver with 32 reports. Other more commonly reported reactions include fatigue, dyspnoea, asthenia, confusion, depression and deep vein thrombosis. [...] All except one of the hepatic reactions involved male patients being treated for prostate cancer, whose ages ranged from 56 to 92 (median: 77) years. Time to onset of liver dysfunction ranged from 4 days to 4 years (median: 4-5 months); only 4 cases had a time to onset under a month.
- Manso G, Thole Z, Salgueiro E, Revuelta P, Hidalgo A (April 2006). "Spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmacovigilance system". Pharmacoepidemiol Drug Saf. 15 (4): 253–9. doi:10.1002/pds.1168. PMID 16294367.
- "Oral contraceptives and liver cancer. Results of the Multicentre International Liver Tumor Study (MILTS)". Contraception. 56 (5): 275–84. November 1997. PMID 9437555.
- Seaman HE, de Vries CS, Farmer RD (2003). "The risk of liver disorders in women prescribed cyproterone acetate in combination with ethinyloestradiol (Dianette): a nested case-control study using the GPRD". Pharmacoepidemiol Drug Saf. 12 (7): 541–50. doi:10.1002/pds.857. PMID 14558177.
- Roe WD, Geschke K, Pease C (December 2009). "Fatal fulminant hepatitis in a chimpanzee (Pan troglodytes) receiving cyproterone acetate". J. Zoo Wildl. Med. 40 (4): 799–802. doi:10.1638/2009-0031.1. PMID 20063830.
- Ralph M. Trüeb (26 February 2013). Female Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 46–. ISBN 978-3-642-35503-5.
- Ramsay ID, Rushton DH (July 1990). "Reduced serum vitamin B12 levels during oral cyproterone-acetate and ethinyl-oestradiol therapy in women with diffuse androgen-dependent alopecia". Clinical and Experimental Dermatology. 15 (4): 277–81. doi:10.1111/j.1365-2230.1990.tb02089.x. PMID 2145099.
- Sadock BJ, Sadock VA (2010). Kaplan and Sadock's Pocket Handbook of Clinical Psychiatry. Lippincott Williams & Wilkins. pp. 582–. ISBN 978-1-60547-264-5.
- Musisi S, Jacobson S (14 April 2015). Brain Degeneration and Dementia in Sub-Saharan Africa. Springer. pp. 60–. ISBN 978-1-4939-2456-1.
- Ní Mhéalóid Á, Cunniffe G (August 2017). "Optic neuritis secondary to antiandrogen therapy". Ir J Med Sci. 186 (3): 565–570. doi:10.1007/s11845-016-1544-1. PMID 28039596.
- Grayling M, Jardine DL, McClintock AD, Spar J, Wilton GN (March 2000). "Symptomatic epidural lipomatosis following cyproterone acetate therapy". Aust N Z J Surg. 70 (3): 233–5. doi:10.1046/j.1440-1622.2000.01793.x. PMID 10765911.
- Mohan D, Taylor R, Mackeith JA (1998). "Cyproterone acetate and striae". International Journal of Psychiatry in Clinical Practice. 2 (2): 147–8. doi:10.3109/13651509809115348. PMID 24946296.
- van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH (April 1990). "Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone". Contraception. 41 (4): 345–52. doi:10.1016/0010-7824(90)90034-S. PMID 2139843.
- Stalvey JR (July 2002). "Inhibition of 3beta-hydroxysteroid dehydrogenase-isomerase in mouse adrenal cells: a direct effect of testosterone". Steroids. 67 (8): 721–31. doi:10.1016/S0039-128X(02)00023-5. PMID 12117620.