Vestibular schwannoma
A vestibular schwannoma (VS) is a benign primary intracranial tumor of the myelin-forming cells of the vestibulocochlear nerve (8th cranial nerve). A type of schwannoma, this tumor arises from the Schwann cells responsible for the myelin sheath that helps keep peripheral nerves insulated.[3] Although it is also called an acoustic neuroma, this is a misnomer for two reasons. First, the tumor usually arises from the vestibular division of the vestibulocochlear nerve, rather than the cochlear division.[4] Second, it is derived from the Schwann cells of the associated nerve, rather than the actual neurons (neuromas).[5]
| Vestibular schwannoma | |
|---|---|
| Other names | Acoustic Neuroma,[1] Acoustic Neurilemoma, Perineural Fibroblastoma, Neurinoma of the acoustic nerve, Neurofibroma of the acoustic nerve, Schwannoma of the acoustic nerve[2] |
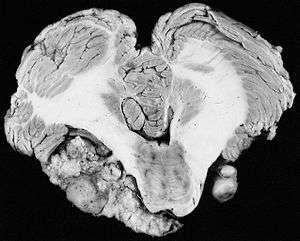 | |
| Bilateral schwannomas in a patient with neurofibromatosis 2 | |
| Specialty | Oncology |
Approximately 2,000 to 3,000 cases are diagnosed each year in the United States (6 to 9 per million persons).[6] Comprehensive studies from Denmark published in 2012 showed an annual incidence of 19-23 per million from 2002 to 2008, and over the last 30 years, the reported incidence has been increasing, until the last decade in which an approximation of the true incidence may have been found.[7] Most recent publications suggest that the incidence of vestibular schwannomas has been rising because of advances in MRI scanning.
Most cases are diagnosed in people between the ages of 30 and 60, and men and women appear to be affected equally.[8] Most vestibular schwannomas occur spontaneously in those without a family history. One confirmed risk factor is a rare genetic mutation called NF2.
The primary symptoms of vestibular schwannoma are unexplained progressive unilateral hearing loss and tinnitus and vestibular (disequilibrium) symptoms. Treatment of the condition is by surgery or radiation and often results in substantial or complete hearing loss in the affected ear. Observation (non-treatment) over time also usually results in hearing loss in the affected ear.
Signs and symptoms
Early symptoms are easily overlooked, sometimes mistaken for the normal changes of aging or attributed to noise exposure earlier in life, often delaying diagnosis. The most prevalent symptoms in patients suffering from vestibular schwannoma is hearing loss (94%), tinnitus (83%) and vertigo (49%).[9]
Hearing loss
The first symptom in 90% of those with an acoustic neuroma is unexplained unilateral sensorineural hearing loss, meaning there is damage to the inner ear (cochlea) or nerve pathways from the inner ear to the brain. It involves a reduction in sound level, speech understanding and hearing clarity. In about 70 percent of cases there is a high frequency pattern of loss. The loss of hearing is usually subtle and worsens slowly, although occasionally a sudden loss of hearing may occur(i.e. sudden deafness). Hearing loss can vary from mild hearing loss to complete deafness.[10]
Tinnitus
Unilateral tinnitus (ringing or hissing in the ears) is also a hallmark symptom of acoustic neuroma. Not all patients with tinnitus have acoustic neuroma and not all AN patients have tinnitus. Most of them do however, both before and after treatment.[11]
Balance
Since the balance portion of the eighth nerve is where the tumor arises, unsteadiness and balance problems or even vertigo (the feeling like the world is spinning), may occur during the growth of the tumor. The remainder of the balance system sometimes compensates for this loss, and, in some cases, no imbalance will be noticed. Balance or vertigo is the third most common symptom in patients with acoustic neuromas (50% incidence). The onset of these may be subtle, like disorientation in dark hallways, and be dismissed as age related decline. These symptoms tend to occur later in the development of the tumor.
Pressure in the ear
Vestibular schwannoma patients sometimes complain of a feeling that their ear is plugged or "full".[11]
Facial weakness or paralysis
Larger tumors can press on the trigeminal nerve (CN V), causing facial numbness and tingling - constantly or intermittently. The facial nerve (CN VII) is rarely affected in the same way; however, due to its proximity to some structures of the inner and middle ear, it can be damaged during radiological treatment or surgical removal of the tumor, particularly in the case of large growths.
At the time some people learn they have an acoustic neuroma, they are also told that this tumor may involve the nerve that controls facial movement. However, it is much more common for treatment, rather than the tumor itself, to damage this nerve, leading to weakness or paralysis of the face. Taste, a sensation that reflects accurately sweet, sour, bitter and bland, is also a function of the facial nerve. Should any of the cranial nerves be damaged or need to be cut during surgery, it is sometimes possible for a neurosurgeon to microsuture the ends together; however, this is a new and very delicate specialist procedure, where long recovery times, incomplete healing and some permanent loss of function are to be expected.
Headache
Recurring headaches are an uncommon symptom, also tending to occur only in cases of larger tumors.
Advanced symptoms
Large tumors may cause disabling and life-threatening symptoms.
Large tumors that compress the adjacent brainstem may affect other local cranial nerves. The glossopharyngeal and vagus nerves are uncommonly involved, but their involvement may lead to altered gag or swallowing reflexes.
Larger tumors may lead to increased intracranial pressure, with its associated symptoms such as headache, vomiting, clumsy gait and mental confusion. This can be a life-threatening complication requiring urgent treatment.[12]
Cause
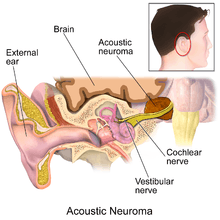
The cause of acoustic neuromas is usually unknown; however there is a growing body of evidence that sporadic defects in tumor suppressor genes may give rise to these tumors in some individuals. In particular, loss or mutation of a tumor suppressor gene on the long arm of chromosome 22 is strongly associated with vestibular schwannomas. Other studies have hinted at exposure to loud noise on a consistent basis. One study has shown a relationship between acoustic neuromas and prior exposure to head and neck radiation, and a concomitant history of having had a parathyroid adenoma (tumor found in proximity to the thyroid gland controlling calcium metabolism). There are even controversies on hand held cellular phones. Whether or not the radiofrequency radiation has anything to do with acoustic neuroma formation, remains to be seen. To date, no environmental factor (such as cell phones or diet) has been scientifically proven to cause these tumors. The Acoustic Neuroma Association (ANA) does recommend that frequent cellular phone users use a hands free device to enable separation of the device from the head.[13]
Although there is an inheritable condition called Neurofibromatosis Type 2 (NF2) which can lead to acoustic neuroma formation in some people, most acoustic neuromas occur spontaneously without any evidence of family history (95%).[8] NF2 occurs with a frequency of 1 in 30,000 to 1 in 50,000 births. The hallmark of this disorder is bilateral acoustic neuromas (an acoustic neuroma on both sides) usually developing in late childhood or early adulthood, frequently associated with other brain and spinal cord tumors.
Pathophysiology
Acoustic neuromas (ARs), the common term for vestibular schwannomas, are neither "acoustic" nor neuromas, since they do not arise from nerve tissue itself - ARs develop from an overproduction of non-neuronal glial (Schwann) cells that support and protect the vestibular (balance) portion of the vestibulocochlear nerve (cranial nerve VIII). ARs are slow-growing local, benign and non-invasive. Progression to malignancy in this kind of tumor is rare. They normally develop gradually over a period of years, expanding at their site of origin roughly 1–2 mm each year; however, up to 50% of such tumors do not grow at all, at least for many years after diagnosis. Tumor growth may be erratic, alternating between periods of relative dormancy or very slow growth and rapid growth. Tumors are typically described as small (less than 1.5 cm), medium (1.5 cm to 2.5 cm), large (2.5 cm to 4 cm),[14] or giant (greater than 4 cm). Tumors are described by a combination of their location and size. An intracanalicular tumor is small and in the internal auditory canal. A cisternal tumor extends outside the auditory canal. A compressive tumor infringes upon the cerebellum or brainstem. Very large tumors may obstruct cerebrospinal fluid drainage.
The tumor may develop within the auditory canal, where the vestibulocochlear nerve which supplies the inner ear penetrates the skull (intracanalicular neuroma) or outside the canal (extra-canalicular neuroma). The vestibulocochlear nerve has two components, the auditory and vestibular portions. Most schwannomas start out as intracanalicular, and growth compresses the nerve against the bony canal, so the first symptoms of the tumor are unilateral sensorineural hearing loss or disturbances in balance. It may also compress the labyrinthine artery (main artery supplying the vestibular apparatus and cochlea of the inner ear) which passes through the auditory canal, resulting in ischemia or infarction ('heart attack' of the ear, resulting in death of the supplied tissue).
As intracanalicular tumors grow, they tend to expand into the cerebellopontine angle (CPA), leading to their characteristic "ice-cream-cone like" appearance on a radiograph. When the tumor expands extracanalicularly, the growth rate often increases, since it is no longer confined by the bony auditory canal. As the schwannoma expands into the CPA, it may infringe on cranial nerve V (controls facial sensation, chewing and swallowing) and cranial nerve VII (controls facial expression and taste). Cranial nerve VIII, along with these two nerves, also passes through the CPA, so more serious or complete hearing loss and episodes of vertigo may occur as the tumor infringes on it there.
When a schwannoma becomes large, it can displace normal brain tissue. The brain is not invaded by the tumor, but the tumor pushes the brain as it enlarges. Vital functions to sustain life can be threatened when large tumors cause severe pressure on the brainstem and cerebellum.
Very large tumors may compress or distort the spinal fluid spaces, resulting in hydrocephalus, with symptoms of headaches, vomiting, nausea, sleepiness and eventually coma.
Diagnosis
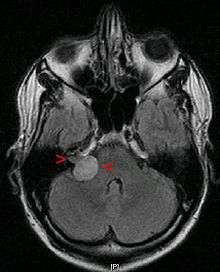

The Gold Standard for diagnosis of vestibular schwannoma is without doubt Gadolinium enhanced magnetic resonance imaging (MRI) yet several examinations may arise suspicion of vestibular schwannomas.
Routine auditory tests may reveal a loss of hearing and speech discrimination (the patient may hear sounds in that ear, but cannot comprehend what is being said). Pure tone audiometry should be performed to effectively evaluate hearing in both ears. In some clinics the clinical criteria for follow up testing for AN is a 15 dB differential in thresholds between ears for three consecutive frequencies.
An auditory brainstem response test (a.k.a. ABR) is a much more cost effective screening alternative to MRI for those at low risk of AN. This test provides information on the passage of an electrical impulse along the circuit from the inner ear to the brainstem pathways. An acoustic neuroma can interfere with the passage of this electrical impulse through the hearing nerve at the site of tumor growth in the internal auditory canal, even when hearing is still essentially normal. This implies the possible diagnosis of an acoustic neuroma when the test result is abnormal. An abnormal auditory brainstem response test should be followed by an MRI. The sensitivity of this test is proportional to the tumor size - the smaller the tumor, the more likely is a false negative result; small tumors within the auditory canal will often be missed. However, since these tumors would usually be watched rather than treated, the clinical significance of overlooking them may be negligible.
Advances in scanning and testing have made possible the identification of small acoustic neuromas (those still confined to the internal auditory canal). MRI using Gadolinium as an enhancing contrast material is the preferred diagnostic test for identifying acoustic neuromas. The image formed clearly defines an acoustic neuroma if it is present and this technique can identify tumors measuring down to 5 millimeters in diameter (the scan spacing).
When an MRI is not available or cannot be performed, a computerized tomography scan (CT scan) with contrast is suggested for patients in whom an acoustic neuroma is suspected. The combination of CT scan and audiogram approach the reliability of MRI in making the diagnosis of acoustic neuroma.[15]
Treatment
There are three treatment options available to a patient. These options are observation, microsurgical removal and radiation (radiosurgery or radiotherapy). Determining which treatment to choose involves consideration of many factors including the size of the tumor, its location, the patient's age, physical health and current symptoms.[15] About 25% of all acoustic neuromas are treated with medical management consisting of a periodic monitoring of the patient's neurological status, serial imaging studies, and the use of hearing aids when appropriate. One of the last great obstacles in the management of acoustic neuromas is hearing preservation and/or rehabilitation after hearing loss. Hearing loss is both a symptom and concomitant risk, regardless of the treatment option chosen. Treatment does not restore hearing already lost, though there are a few rare cases of hearing recovery reported.
A diagnosis of NF2 related bilateral acoustic neuromas creates the possibility of complete deafness if the tumors are left to grow unchecked. Preventing or treating the complete deafness that may befall individuals with NF2 requires complex decision making. The trend at most academic U.S. medical centers is to recommend treatment for the smallest tumor which has the best chance of preserving hearing. If this goal is successful, then treatment can also be offered for the remaining tumor. If hearing is not preserved at the initial treatment, then usually the second tumor, in the only-hearing ear, is just observed. If it shows continued growth and becomes life-threatening, or if the hearing is lost over time as the tumor grows, then treatment is undertaken. This strategy has the highest chance of preserving hearing for the longest time possible.[16]
Observation
Since acoustic neuromas tend to be slow-growing and are benign tumors, careful observation over a period of time may be appropriate for some patients. When a small tumor is discovered in an older patient, observation to determine the growth rate of the tumor may be indicated if serious symptoms are not present. There is now good evidence from large observational studies that suggest many small tumors in older individuals do not grow, thus allowing tumors with no growth to be observed successfully. If the tumor grows, treatment may become necessary. Another example of a group of patients for whom observation may be indicated includes patients with a tumor in their only hearing or better hearing ear, particularly when the tumor is of a size that hearing preservation with treatment would be unlikely. In this group of patients, MRI is used to follow the growth pattern. Treatment is recommended if either the hearing is lost or the tumor size becomes life-threatening, thus allowing the patient to retain hearing for as long as possible.[17]
Current studies suggest surgeons should observe small acoustic neuromas (those 1.5 cm or less).[7]
Over a period of 10 years of observation with no treatment, 45% of patients with small tumors (and therefore minimal symptoms) lose functional hearing on the affected side; this percentage is considerably higher than that for patients actively treated with hearing-preserving microsurgery or radiosurgery.
Surgery
The goals of surgery are to control the tumor, and preserve function of the involved nerves (i.e. those involved in facial musculature and hearing). Preservation of hearing is an important goal for patients who present with functional hearing.[17] Surgery cannot restore hearing already lost.
Microsurgical tumor removal can be done at one of three levels: subtotal removal, near total removal or total tumor removal. Many tumors can be entirely removed by surgery. Microsurgical techniques and instruments, along with the operating microscope, have greatly reduced the surgical risks of total tumor removal. Subtotal removal is indicated when anything further risks life or neurological function. In these cases the residual tumor should be followed for risk of growth (approximately 35%). If the residual grows further, treatment will likely be required. Periodic MRI studies are important to follow the potential growth rate of any tumor. Near total tumor removal is used when small areas of the tumor are so adherent to the facial nerve that total removal would result in facial weakness. The piece left is generally less than 1% of the original and poses a risk of regrowth of approximately 3%.
There are three main surgical approaches for the removal of an acoustic neuroma: translabyrinthine, retrosigmoid/sub-occipital and middle fossa. The approach used for each individual person is based on several factors such as tumor size, location, skill and experience of the surgeon, and whether hearing preservation is a goal. Each of the surgical approaches has advantages and disadvantages in terms of ease of tumor removal, likelihood of preservation of facial nerve function and hearing, and post-operative complications.[18]
During surgery, intraoperative neurophysiological monitoring of the facial, acoustic and lower cranial nerves can reduce the risk of injury.[19][20][21] In particular, following the 1991 NIH National institutes of Health Acoustic Neuroma Consensus Panel, the use of facial nerve monitoring has become a standard practice in the United States to reduce the risk of facial paralysis.[22]
With massive tumors that compress the brainstem and cerebellum, staged surgical approaches or subtotal surgical resection followed by stereotactic radiosurgery may reduce the risks to life, brain and cranial nerves.[23][24]
Translabyrinthine approach
The translabyrinthine approach may be preferred by the surgical team when the patient has no useful hearing, or when an attempt to preserve hearing would be impractical. The incision for this approach is located behind the ear and allows excellent exposure of the internal auditory canal and tumor. Since the incision goes directly through the inner ear, this results in permanent and complete hearing loss in that ear. Many patients with medium to large ANs have no functional hearing in the ear anyway, so this may not be an issue. The surgeon has the advantage of knowing the location of the facial nerve prior to tumor dissection and removal. Any size tumor can be removed with this approach and this approach affords the least likelihood of long-term postoperative headaches.[25]
Retrosigmoid/sub-occipital approach
The incision for this approach is located in a slightly different location. This approach creates an opening in the skull behind the mastoid part of the ear, near the back of the head on the side of the tumor. The surgeon exposes the tumor from its posterior (back) surface, thereby getting a very good view of the tumor in relation to the brainstem. When removing large tumors through this approach, the facial nerve can be exposed by early opening of the internal auditory canal. Any size tumor can be removed with this approach. One of the main advantages of the retrosigmoid approach is the possibility of preserving hearing.[26] For small tumors, a disadvantage lies in the risk of long-term postoperative headache.[27]
Middle fossa approach
This approach is in a slightly different incision location and is utilized primarily for the purpose of hearing preservation in patients with small tumors, typically confined to the internal auditory canal. A small window of bone is removed above the ear canal to allow exposure of the tumor from the upper surface of the internal auditory canal, preserving the inner ear structures.[27]
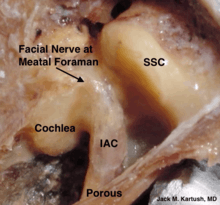
Complications due to surgery
Cancers (radiotherapy)
There are documented incidences of new malignant gliomas and malignant progression of ANs after focused radiotherapy using either SRS or FRT for benign intracranial lesions.
Tinnitus
Most patients present with tinnitus before treatment, and also have it after treatment. About one in 5 patients without tinnitus acquire it, and for about 2 in 5 with tinnitus it resolves or decreases.
Hearing loss
While formerly, preservation of hearing during treatment was very unlikely, the newer techniques of microsurgery and stereotactic radiotherapy have enabled the preservation of functional hearing in the majority of cases. Overall, 60-66% of persons treated for AR preserve their hearing. Likelihood of preserving hearing is correlated with better hearing pre-treatment, and smaller size of tumor. If preservation of hearing is an important goal, the choice of surgical approach may be different. Even in those with functional hearing following surgery or radiotherapy, hearing may decline for years afterward.
Tumor regrowth
Tumor regrowth occurs in 1-3% of cases treated surgically, and 14% in cases treated with radiation. Likelihood of regrowth is proportional to the bulk of tumor remaining in case of surgery, and inversely proportional to radiation dose in case of radiotherapy. In case retreatment with surgery following radiation was required, the rate of complications was from 19.4%[29] to 27%[30] in two different studies, because the tumor tends to fuse to the nerve.
Facial nerve damage
In the 2012 Acoustic Neuroma Association patient survey, 29% of the respondents reported facial weakness or paralysis, some of which were pre- and some were post-treatment. This represents a significant improvement from the 1998 Acoustic Neuroma Association patient survey of post-treatment acoustic neuroma patients, which revealed that at the time they completed the survey, only 59% were satisfied with the appearance of their face. Treatment for an acoustic neuroma may damage the facial nerve – either with surgery or radiation. It is usually possible, however, to preserve some degree of facial function even in cases where the nerve is extensively involved. For those with partial nerve regeneration, in whom some facial weakness remains, non-surgical facial rehabilitation therapies also may be beneficial.[31]
Taste disturbance and mouth dryness
Taste disturbance and mouth dryness are frequent for a few weeks following surgery. In a few patients this disturbance is longer or permanent.
Headaches
Head pain is expected in most patients immediately after acoustic neuroma surgery (acute phase) because of the incision, variations in cerebrospinal fluid pressure, muscle pain, or even meningitic pain. It typically responds to appropriate medications and resolves within several weeks. Headache that persists for months or even years after surgery (chronic phase) can be debilitating and may be an under-appreciated complication of acoustic neuroma treatment. In patients who experience chronic headaches, the pain often persists for prolonged periods of time, and does not always respond well to various medical and surgical treatments. The exact prevalence and causes of chronic postoperative headache (POH) are elusive. After surgical treatment of acoustic neuroma, the reported incidence of headache in the 2012 Acoustic Neuroma Association patient survey has ranged from 0% to 35% depending on the type of surgical approach, technique used and reporting interval since surgery. Frequent and severe post-operative headaches have been more often associated with the sub-occipital/retrosigmoid approach than the translabyrinthine or middle fossa approaches.[32][33]
Balance
Essentially everyone who has been treated for an acoustic neuroma experiences difficulty with balance and/or dizziness to some degree. For some, this instability may be mild and noticeable only in certain circumstances, such as ambulating with head movements, or walking in the dark. For others, there may be difficulty returning to work, or even performing regular daily activities such as driving, shopping, house work and even working on a computer.[34]
Paralysis and death
In rare cases where large tumors infringe on the brainstem which controls motor nerves, with or without surgery, paralysis or death can result. This occurs in less than 1% of large tumors.
Radiation
Another treatment option for an acoustic neuroma is radiation. Stereotactic radiation can be delivered as single fraction stereotactic radiosurgery (SRS) or as multi-session fractionated stereotactic radiotherapy (FSR). Both techniques are performed in the outpatient setting, not requiring general anesthesia or a hospital stay. The purpose of these techniques is to arrest the growth of the tumor. This treatment has not been well studied and thus it is unclear if it is better than observation or surgery.[35]
All types of radiation therapy for acoustic neuromas may result in "tumor control" in which the tumor cells die and necrosis occurs. Tumor control means that the tumor growth may slow or stop and, in some cases, the tumor may shrink in size. Acoustic neuroma tumors have been completely eliminated by radiation treatments in almost no cases. In other words, radiation cannot remove the tumor like microsurgery would. Tumors under 2.5 - 3.0 cm, without significant involvement of the brainstem, are more favorable for radiation treatment. Side effects can occur when the brainstem is irradiated and in some cases of large tumors, radiation is suggested against.
In single dose treatments, hundreds of small beams of radiation are aimed at the tumor. This results in a concentrated dose of radiation to the tumor and avoids exposure of surrounding brain tissues to the radiation. Many patients have been successfully treated this way. Facial weakness or numbness, in the hands of experienced radiation physicians, occurs in only a small percent of cases. Hearing can be preserved in some cases.
The multi-dose treatment, FSR, delivers smaller doses of radiation over a period of time, requiring the patient to return to the treatment location on a daily basis, from 3 to 30 times, generally over several weeks. Each visit lasts a few minutes and most patients are free to go about their daily business before and after each treatment session. Early data indicates that FSR may result in better hearing preservation when compared to single-session SRS.
Radiated patients require lifetime follow-up with MRI scans. Follow-up after SRS and FSR typically involves an MRI scan and audiogram at six months, one year, then yearly for several years, then every second or third year indefinitely to make sure the tumor does not start to grow again. Patients should understand there have been rare reports of malignant degeneration (a benign tumor becoming malignant) after radiotherapy. In some cases the tumor does not die and continues to grow. In those instances, another treatment is necessary - either microsurgery or sometimes another dose of radiation.
Studies are beginning to appear for the other modalities. All of the techniques use computers to create three dimensional models of the tumor and surrounding neural structures. Radiation physicists then create dosimetry maps showing the level of radiation to be received by the tumor and the normal tissues. Surgeons, radiation therapists and physicists then modify the dosimetry to maximize tumor doses and minimize radiation toxicity to surrounding normal tissues. Treatments generally last 30–60 minutes. Just like for surgery, the experience of the team in treating acoustic neuromas with all modalities (surgery and radiation) can affect outcomes.
There are a multitude of studies supporting short-term (<5 yrs.) and longer-term (over 10 yrs.) tumor control with radiation. Unfortunately, as is the case with microsurgical studies, most have inconsistent follow-up to draw definitive conclusions.[36]
Epidemiology
Vestibular schwannoma is a rare condition: incident rate in the U.S. in 2010 was 11/1,000,000 persons, mean age 53. Occurrence was equally distributed versus age, gender and laterality. In patients with unilateral hearing loss, only about 1 in 1000 has acoustic neuroma.
Notable people
American actor, director, humanitarian, social activist and film producer Mark Ruffalo was diagnosed with vestibular schwannoma in 2001 which resulted in a period of partial facial paralysis.[37] He recovered from the paralysis; however, he became deaf in his left ear as a result of the tumor.[38]
Guitarist/composer/producer David Torn was diagnosed with an acoustic neuroma in 1992. It required intricate surgery that left him deaf in the right ear and burdened by many other health obstacles.[39]
American actress and designer Tara Subkoff was diagnosed with schwannoma in 2009. Successfully underwent surgery, but was left with permanent nerve damage and deafness in right ear.[40]
Tionne Watkins, better known under her stage name T-Boz, R&B singer from the R&B/Hip Hop group TLC was diagnosed with a strawberry-sized acoustic neuroma on her vestibular nerve in 2006.[41] Many physicians refused to remove the tumor due to her sickle-cell-related complications, leaving her alternatives grim. Ultimately, she underwent surgery at Cedars-Sinai Hospital in Los Angeles.[42]
See also
Notes
- "Vestibular Schwannoma (Acoustic Neuroma) and Neurofibromatosis". NIDCD. 18 August 2015. Retrieved 13 August 2017.
- "Acoustic Neuroma". NORD. Retrieved 16 July 2018.
- "Acoustic Neuroma". NHS Choices. Retrieved 30 August 2013.
- Page 67 in: Sunjay Parmar (2017). Neurology: A Visual Approach. CRC Press. ISBN 9781498782074.
- Page 31 in: Fred F. Ferri (2013). BOPOD - Ferri's Clinical Advisor. Elsevier Health Sciences. ISBN 9780323084314.
- "Acoustic neuroma". Consensus Statement. 9 (4): 1–24. 1991. PMID 1840823.
- Stangerup, Sven-Eric; Caye-Thomasen, Per (2012). "Epidemiology and Natural History of Vestibular Schwannomas". Otolaryngologic Clinics of North America. 45 (2): 257–68, vii. doi:10.1016/j.otc.2011.12.008. PMID 22483814.
- ANA Overview 2015, p. 3.
- Kentala, E.; Pyykkö, I. (January 2001). "Clinical picture of vestibular schwannoma". Auris, Nasus, Larynx. 28 (1): 15–22. doi:10.1016/S0385-8146(00)00093-6. ISSN 0385-8146. PMID 11137358.
- ANA Hearing 2013, p. 1.
- ANA Overview 2015, p. 4.
- ANA Overview 2015, p. 2.
- ANA Overview 2015, p. 10.
- ANA Overview 2015, p. 1.
- ANA Overview 2015, p. 5.
- ANA Overview 2015, pp. 10–11.
- ANA Overview 2015, p. 6.
- ANA Overview 2015, p. 7.
- Kartush JM: Electroneurography and Intraoperative Facial Monitoring in Contemporary Neurotology. Otolaryngology–Head and Neck Surgery, Vol. l0l, No. 4, pp. 496-503, October, l989
- Kartush JM, Bouchard KR: Intraoperative Facial Nerve Monitoring: Otology, Neurotology and Skull Base Surgery. Neuromonitoring in Otology and Head and Neck Surgery. J. M. Kartush, K. R. Bouchard (eds.). Raven Press, New York, ch. 5, pp. 99-l20, l992
- Kircher ML, Kartush JM. Pitfalls in intraoperative nerve monitoring during vestibular schwannoma surgery. Neurosurg Focus 2012;33(3):E5
- NIH Acoustic Neuroma Consensus Statement Online 1991 Dec 11-13;9(4):1-24. Reprinted: National Institutes of Health Consensus Development Conference Statement on Acoustic Neuroma, December 11–13, 1991. Arch Neurol 1994;51(2):201-207
- Patni A, Kartush J: Staged resection of large acoustic neuromas. Otolaryng H N Surg, Vol 132/1 pp 11-19, 2005
- Porter RG, LaRouere MJ, Kartush JM, Bojrab DI, Pieper DR: Improved facial nerve outcomes using an evolving treatment method for large acoustic neuromas: Otol Neurotol. 2013 Feb;34 (2):304-10
- ANA Overview 2015, pp. 7–8.
- Kartush JM, Telian SA, Graham MD, Kemink JL: Anatomic Basis for Labyrinthine Preservation During Posterior Fossa Acoustic Tumor Surgery. Laryngoscope, Vol. 96, pp. l024-l028, September, l986
- ANA Overview 2015, p. 8.
- Kartush JM, Kemink JL, Graham MD: The Arcuate Eminence--Topographic Orientation in Middle Cranial Fossa Surgery. Annals of Otology, Rhinology, & Laryngology, Vol. 94, pp. 25-28, January–February, l985
- Nonaka, Yoichi; Fukushima, Takanori; Watanabe, Kentaro; Friedman, Allan H.; Cunningham, Calhoun D.; Zomorodi, Ali R. (2016). "Surgical management of vestibular schwannomas after failed radiation treatment". Neurosurgical Review. 39 (2): 303–12, discussion 312. doi:10.1007/s10143-015-0690-7. PMID 26782633.
- Wise, Stephanie C.; Carlson, Matthew L.; Tveiten, Øystein Vesterli; Driscoll, Colin L.; Myrseth, Erling; Lund-Johansen, Morten; Link, Michael J. (2016). "Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery". The Laryngoscope. 126 (11): 2580–2586. doi:10.1002/lary.25943. PMID 27107262.
- ANA Facial 2015, p. 1.
- ANA Headache 2013, pp. 1–2.
- Idleman & Associates 2012, p. .
- ANA Balance 2013, p. .
- Muzevic, Dario; Legcevic, Jelena; Splavski, Bruno; Cayé-Thomasen, Per; Muzevic, Dario (2014). "Stereotactic radiotherapy for vestibular schwannoma". The Cochrane Database of Systematic Reviews. 12 (12): CD009897. doi:10.1002/14651858.CD009897.pub2. PMID 25511415.
- ANA Overview 2015, pp. 8–10.
- Radar, Dotson (May 9, 2004). "I Wouldn't Give Any Of It Back". Parade. Archived from the original on September 30, 2007. Retrieved September 20, 2007.
- Hiatt, Brian (May 4, 2015). "The Hulk: The Last Angry Man". Rolling Stone. Wenner Media, Ltd. Retrieved May 5, 2015.
- Prasad, Anil (1995). "Innerviews: David Torn - Fate is not completely decided". Retrieved October 18, 2017.
- Blasberg, Derek (2010-04-17). "Tara Subkoff: 'I survived a brain tumor!'". Harpers Bazaar. Retrieved 2015-10-20.
- http://www.cbsnews.com/news/t-bozs-brain-tumor-battle/
- Atlanta Entertainment News. "Tionne "T-Boz" Watkins of TLC Discusses Brain Tumor & Sickle Cell". StraightFromTheA.com. Retrieved 2012-04-23.
References
- Acoustic Neuroma Basic Overview. Acoustic Neuroma Association. March 2015.
- Hearing Loss Rehabilitation For Acoustic Neuroma Patients. Acoustic Neuroma Association. January 2013.
- Facial Nerve & Acoustic Neuroma: Possible Damage & Rehabilitation. Acoustic Neuroma Association. July 2015.
- Headache Associated with Acoustic Neuroma Treatment. Acoustic Neuroma Association. November 2013.
- Improving Balance Associated with Acoustic Neuroma. Acoustic Neuroma Association. September 2013.
- Idleman & Associates (2012). 2012 ANA Patient Survey. Acoustic Neuroma Association.
- Idleman & Associates (2014). 2014 Report on ANA Patient Database. Acoustic Neuroma Association.
Further reading
- Evans, D. Gareth R.; Moran, Anthony; King, Andrew; Saeed, S.; Gurusinghe, Nihal; Ramsden, Richard (2005). "Incidence of Vestibular Schwannoma and Neurofibromatosis 2 in the North West of England over a 10-year Period: Higher Incidence than Previously Thought". Otology & Neurotology. 26 (1): 93–7. doi:10.1097/00129492-200501000-00016. PMID 15699726.
- Shin, Masahiro; Ueki, Keisuke; Kurita, Hiroki; Kirino, Takaaki (2002). "Malignant transformation of a vestibular schwannoma after gamma knife radiosurgery". The Lancet. 360 (9329): 309–10. doi:10.1016/S0140-6736(02)09521-1. PMID 12147377.
- Samii, Madjid; Gerganov, Venelin; Samii, Amir (2006). "Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients". Journal of Neurosurgery. 105 (4): 527–35. doi:10.3171/jns.2006.105.4.527. PMID 17044553.
- Pollock, Bruce E.; Driscoll, Colin L.W.; Foote, Robert L.; Link, Michael J.; Gorman, Deborah A.; Bauch, Christopher D.; Mandrekar, Jayawant N.; Krecke, Karl N.; Johnson, Craig H. (2006). "Patient Outcomes after Vestibular Schwannoma Management: A Prospective Comparison of Microsurgical Resection and Stereotactic Radiosurgery". Neurosurgery. 59 (1): 77–85, discussion 77–85. doi:10.1227/01.NEU.0000219217.14930.14. PMID 16823303.
- Prasad, Dheerendra; Steiner, Melita; Steiner, Ladislau (2000). "Gamma surgery for vestibular schwannoma". Journal of Neurosurgery. 92 (5): 745–59. doi:10.3171/jns.2000.92.5.0745. PMID 10794287.
- Stangerup, Sven-Eric; Caye-Thomasen, Per; Tos, Mirko; Thomsen, Jens (2006). "The natural history of vestibular schwannoma". Otology & Neurotology. 27 (4): 547–52. doi:10.1097/01.mao.0000217356.73463.e7. PMID 16791048.
- Kanzaki, Jin; Tos, Mirko; Sanna, Mario; Moffat, David A. (2003). "New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma". Otology & Neurotology. 24 (4): 642–8, discussion 648–9. doi:10.1097/00129492-200307000-00019. PMID 12851559.
- Tos, Mirko; Stangerup, Sven-Eric; Cayé-Thomasen, Per; Tos, Tina; Thomsen, Jens (2004). "What Is the Real Incidence of Vestibular Schwannoma?". Archives of Otolaryngology–Head & Neck Surgery. 130 (2): 216–20. doi:10.1001/archotol.130.2.216. PMID 14967754.
External links
| Classification | |
|---|---|
| External resources |
| Wikimedia Commons has media related to Vestibular schwannoma. |
- Vestibular schwannoma at Curlie