Fetal alcohol spectrum disorder
Fetal alcohol spectrum disorders (FASDs) are a group of conditions that can occur in a person whose mother drank alcohol during their pregnancy.[1] Problems may include an abnormal appearance, short height, low body weight, small head size, poor coordination, low intelligence, behavior problems, and problems with hearing or seeing.[1][2] Those affected are more likely to have trouble in school, legal problems, participate in high-risk behaviors, and have trouble with alcohol or other drugs.[7] The most severe form of the condition is known as fetal alcohol syndrome (FAS).[1] Other types include partial fetal alcohol syndrome (pFAS), alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBD).[1][8] Some accept only FAS as a diagnosis, seeing the evidence as inconclusive with respect to other types.[9]
| Fetal alcohol spectrum disorders | |
|---|---|
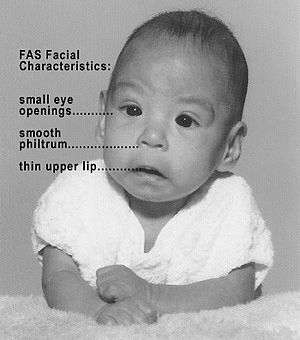 | |
| Baby with fetal alcohol syndrome. | |
| Specialty | Psychiatry, pediatrics, toxicology |
| Symptoms | Abnormal appearance, short height, low body weight, small head size, poor coordination, low intelligence, behavior problems[1][2] |
| Duration | Long term[1][3] |
| Types | Fetal alcohol syndrome, partial fetal alcohol syndrome, alcohol-related neurodevelopmental disorder, alcohol-related birth defects[1] |
| Causes | Drinking alcohol during pregnancy[1] |
| Diagnostic method | Based on symptoms[1] |
| Prevention | Avoiding drinking alcohol during pregnancy[4] |
| Treatment | Parent-child interaction therapy, efforts to modify child behavior, possibly medications[5] |
| Frequency | 1–5% (US, EU)[6] |
Fetal alcohol spectrum disorders are caused by drinking alcohol during pregnancy.[1] Surveys from the United States have found about 10% of pregnant women have drunk alcohol in the last month, and 20% to 30% drank at some point during the pregnancy.[10] About 3.6% of pregnant American women are alcoholics.[11] The risk of problems depends on the amount consumed and the frequency of consumption as well as when during pregnancy the alcohol is consumed.[10] Other risk factors include an older mother, smoking, and poor diet.[12][10] There is no known safe amount or safe time to drink during pregnancy.[1][13] While drinking small amounts of alcohol does not cause abnormalities in the face, it may cause behavioral issues.[11] Alcohol crosses the blood brain barrier and both directly and indirectly affects a developing baby.[14] Diagnosis is based on signs and symptoms in the person.[1]
Fetal alcohol spectrum disorders are preventable by avoiding alcohol.[4] For this reason, medical authorities recommend no alcohol during pregnancy or while trying to become pregnant.[15][16][17] While the condition is permanent, treatment can improve outcomes.[1][3] Interventions may include parent-child interaction therapy, efforts to modify child behavior, and possibly medications.[5]
FASD is estimated to affect between 1% and 5% of people in the United States and Western Europe.[6] FAS is believed to occur in between 0.2 and 9 per 1000 live births in the United States.[6] In South Africa, some populations have rates as high as 9%.[8] The negative effects of alcohol during pregnancy have been described since ancient times.[8] The lifetime cost per child with FAS was $2,000,000 in 2002 in the US.[6] The term fetal alcohol syndrome was first used in 1973.[8]
Types
FASDs encompass a range of physical and neurodevelopmental problems that can result from prenatal alcohol exposure.[1] The most severe condition is called fetal alcohol syndrome (FAS),[1] which refers to individuals who have a specific set of birth defects and neurodevelopmental disorders characteristic of the diagnosis.[18]
Some accept only FAS as a diagnosis, seeing the evidence as inconclusive with respect to other types.[9] Partial fetal alcohol syndrome (pFAS) refers to individuals with a known, or highly suspected, history of prenatal alcohol exposure who have alcohol-related physical and neurodevelopmental deficits that do not meet the full criteria for FAS.[18] The subtypes of pFAS are alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBD).[18] In addition to FAS, pFAS, ARND, and ARBD, any other conditions believed to be related to prenatal alcohol exposure, such as spontaneous abortion and sudden infant death syndrome (SIDS), are also considered to be on the spectrum of related disorders.[18] It is unclear as of 2017 if identifying a FASD-related conditions benefits the individual.[9]
Signs and symptoms

The key of FASD can vary between individuals exposed to alcohol during pregnancy. While consensus exists for the definition and diagnosis of FAS, minor variations among the systems lead to differences in definitions and diagnostic cut-off criteria for other diagnoses across the FASD continuum.
The central nervous system damage criteria particularly lack clear consensus. A working knowledge of the key features is helpful in understanding FASD diagnoses and conditions, and each is reviewed with attention to similarities and differences across the four diagnostic systems. More than 400 problems, however, can occur with FASD.[19]
Growth
In terms of FASD, growth deficiency is defined as significantly below average height, weight or both due to prenatal alcohol exposure, and can be assessed at any point in the lifespan. Growth measurements must be adjusted for parental height, gestational age (for a premature infant), and other postnatal insults (e.g., poor nutrition), although birth height and weight are the preferred measurements.[20] Deficiencies are documented when height or weight falls at or below the 10th percentile of standardized growth charts appropriate to the population.[21] Prenatal or postnatal presentation of growth deficits can occur, but are most often postnatal.[22]
Criteria for FASD are least specific in the IOM diagnostic system ("low birth weight..., decelerating weight not due to nutrition..., [or] disproportional low weight to height" p. 4 of executive summary),[16] while the CDC and Canadian guidelines use the 10th percentile as a cut-off to determine growth deficiency.[2][23] The "4-Digit Diagnostic Code" allows for mid-range gradations in growth deficiency (between the 3rd and 10th percentiles) and severe growth deficiency at or below the 3rd percentile.[20] Growth deficiency (at severe, moderate, or mild levels) contributes to diagnoses of FAS and pFAS, but not ARND or static encephalopathy.
Growth deficiency is ranked as follows by the "4-Digit Diagnostic Code":[20]
- Severe: Height and weight at or below the 3rd percentile.
- Moderate: Either height or weight at or below the 3rd percentile, but not both.
- Mild: Either height or weight or both between the 3rd and 10th percentiles.
- None: Height and weight both above the 10th percentile.
In the initial studies that discovered FAS, growth deficiency was a requirement for inclusion in the studies; thus, all the original people with FAS had growth deficiency as an artifact of sampling characteristics used to establish criteria for the syndrome. That is, growth deficiency is a key feature of FASD because growth deficiency was a criterion for inclusion in the study that defined FAS. This suggests growth deficiency may be less critical for understanding the disabilities of FASD than the neurobehavioral sequelae to the brain damage.[16]
Facial features
Several characteristic craniofacial abnormalities are often visible in individuals with FAS.[24] The presence of FAS facial features indicates brain damage, although brain damage may also exist in their absence. FAS facial features (and most other visible, but non-diagnostic, deformities) are believed to be caused mainly during the 10th and 20th week of gestation.[25]
Refinements in diagnostic criteria since 1975 have yielded three distinctive and diagnostically significant facial features known to result from prenatal alcohol exposure and distinguishes FAS from other disorders with partially overlapping characteristics.[26][27] The three FAS facial features are:
- A smooth philtrum: The divot or groove between the nose and upper lip flattens with increased prenatal alcohol exposure.
- Thin vermilion: The upper lip thins with increased prenatal alcohol exposure.
- Small palpebral fissures: Eye width decreases with increased prenatal alcohol exposure.
Measurement of FAS facial features uses criteria developed by the University of Washington. The lip and philtrum are measured by a trained physician with the Lip-Philtrum Guide,[28] a five-point Likert Scale with representative photographs of lip and philtrum combinations ranging from normal (ranked 1) to severe (ranked 5). Palpebral fissure length (PFL) is measured in millimeters with either calipers or a clear ruler and then compared to a PFL growth chart, also developed by the University of Washington.[29]
Ranking FAS facial features is complicated because the three separate facial features can be affected independently by prenatal alcohol. A summary of the criteria follows:[20][30]
- Severe: All three facial features ranked independently as severe (lip ranked at 4 or 5, philtrum ranked at 4 or 5, and PFL two or more standard deviations below average).
- Moderate: Two facial features ranked as severe and one feature ranked as moderate (lip or philtrum ranked at 3, or PFL between one and two standard deviations below average).
- Mild: A mild ranking of FAS facial features covers a broad range of facial feature combinations:
- Two facial features ranked severe and one ranked within normal limits,
- One facial feature ranked severe and two ranked moderate, or
- One facial feature ranked severe, one ranked moderate and one ranked within normal limits.
- None: All three facial features ranked within normal limits.
Central nervous system
Central nervous system (CNS) damage is the primary feature of any FASD diagnosis. Prenatal alcohol exposure, which is classified as a teratogen, can damage the brain across a continuum of gross to subtle impairments, depending on the amount, timing, and frequency of the exposure as well as genetic predispositions of the fetus and mother.[16][31] While functional abnormalities are the behavioral and cognitive expressions of the FASD disability, CNS damage can be assessed in three areas: structural, neurological, and functional impairments.
All four diagnostic systems allow for assessment of CNS damage in these areas, but criteria vary. The IOM system requires structural or neurological impairment for a diagnosis of FAS, but also allows a "complex pattern" of functional anomalies for diagnosing PFAS and ARND.[16] The "4-Digit Diagnostic Code" and CDC guidelines allow for a positive CNS finding in any of the three areas for any FASD diagnosis, but functional anomalies must measure at two standard deviations or worse in three or more functional domains for a diagnosis of FAS, PFAS, and ARND.[20][23] The "4-Digit Diagnostic Code" also allows for an FASD diagnosis when only two functional domains are measured at two standard deviations or worse.[20] The "4-Digit Diagnostic Code" further elaborates the degree of CNS damage according to four ranks:
- Definite: Structural impairments or neurological impairments for FAS or static encephalopathy.
- Probable: Significant dysfunction of two standard deviations or worse in three or more functional domains.
- Possible: Mild to moderate dysfunction of two standard deviations or worse in one or two functional domains or by judgment of the clinical evaluation team that CNS damage cannot be dismissed.
- Unlikely: No evidence of CNS damage.
Structural
Structural abnormalities of the brain are observable, physical damage to the brain or brain structures caused by prenatal alcohol exposure. Structural impairments may include microcephaly (small head size) of two or more standard deviations below the average, or other abnormalities in brain structure (e.g., agenesis of the corpus callosum, cerebellar hypoplasia).[16]
Microcephaly is determined by comparing head circumference (often called occipitofrontal circumference, or OFC) to appropriate OFC growth charts.[21] Other structural impairments must be observed through medical imaging techniques by a trained physician. Because imaging procedures are expensive and relatively inaccessible to most people, diagnosis of FAS is not frequently made via structural impairments, except for microcephaly.
Evidence of a CNS structural impairment due to prenatal alcohol exposure will result in a diagnosis of FAS, and neurological and functional impairments are highly likely.[2][16][20][23]
During the first trimester of pregnancy, alcohol interferes with the migration and organization of brain cells, which can create structural deformities or deficits within the brain.[32] During the third trimester, damage can be caused to the hippocampus, which plays a role in memory, learning, emotion, and encoding visual and auditory information, all of which can create neurological and functional CNS impairments as well.[33]
As of 2002, there were 25 reports of autopsies on infants known to have FAS. The first was in 1973, on an infant who died shortly after birth.[34] The examination revealed extensive brain damage, including microcephaly, migration anomalies, callosal dysgenesis, and a massive neuroglial, leptomeningeal heterotopia covering the left hemisphere.[35]
In 1977, Dr. Clarren described a second infant whose mother was a binge drinker. The infant died ten days after birth. The autopsy showed severe hydrocephalus, abnormal neuronal migration, and a small corpus callosum (which connects the two brain hemispheres) and cerebellum.[35] FAS has also been linked to brainstem and cerebellar changes, agenesis of the corpus callosum and anterior commissure, neuronal migration errors, absent olfactory bulbs, meningomyelocele, and porencephaly.[35]
Neurological
When structural impairments are not observable or do not exist, neurological impairments are assessed. In the context of FASD, neurological impairments are caused by prenatal alcohol exposure which causes general neurological damage to the central nervous system (CNS), the peripheral nervous system, or the autonomic nervous system. A determination of a neurological problem must be made by a trained physician, and must not be due to a postnatal insult, such as a high fever, concussion, traumatic brain injury, etc.
All four diagnostic systems show virtual agreement on their criteria for CNS damage at the neurological level, and evidence of a CNS neurological impairment due to prenatal alcohol exposure will result in a diagnosis of FAS or pFAS, and functional impairments are highly likely.[2][16][20][23]
Neurological problems are expressed as either hard signs, or diagnosable disorders, such as epilepsy or other seizure disorders, or soft signs. Soft signs are broader, nonspecific neurological impairments, or symptoms, such as impaired fine motor skills, neurosensory hearing loss, poor gait, clumsiness, poor eye-hand coordination. Many soft signs have norm-referenced criteria, while others are determined through clinical judgment. "Clinical judgment" is only as good as the clinician, and soft signs should be assessed by either a pediatric neurologist, a pediatric neuropsychologist, or both.
Functional
When structural or neurological impairments are not observed, all four diagnostic systems allow CNS damage due to prenatal alcohol exposure to be assessed in terms of functional impairments.[2][16][20][23] Functional impairments are deficits, problems, delays, or abnormalities due to prenatal alcohol exposure (rather than hereditary causes or postnatal insults) in observable and measurable domains related to daily functioning, often referred to as developmental disabilities. There is no consensus on a specific pattern of functional impairments due to prenatal alcohol exposure[16] and only CDC guidelines label developmental delays as such,[23] so criteria (and FASD diagnoses) vary somewhat across diagnostic systems.
The four diagnostic systems list various CNS domains that can qualify for functional impairment that can determine an FASD diagnosis:
- Evidence of a complex pattern of behavior or cognitive abnormalities inconsistent with developmental level in the following CNS domains – Sufficient for a pFAS or ARND diagnosis using IOM guidelines[16]
- Performance at two or more standard deviations on standardized testing in three or more of the following CNS domains – Sufficient for an FAS, pFAS or static encephalopathy diagnosis using 4-Digit Diagnostic Code[20]
- Executive functioning, memory, cognition, social/adaptive skills, academic achievement, language, motor skills, attention, activity level
- General cognitive deficits (e.g., IQ) at or below the 3rd percentile on standardized testing – Sufficient for an FAS diagnosis using CDC guidelines[23]
- Performance at or below the 16th percentile on standardized testing in three or more of the following CNS domains – Sufficient for an FAS diagnosis using CDC guidelines[23]
- Cognition, executive functioning, motor functioning, attention and hyperactive problems, social skills, sensory processing disorder, social communication, memory, difficulties responding to common parenting practices
- Performance at two or more standard deviations on standardized testing in three or more of the following CNS domains – Sufficient for an FAS diagnosis using Canadian guidelines
- Cognition, communication, academic achievement, memory, executive functioning, adaptive behavior, motor skills, social skills, social communication
Related signs
Other conditions may commonly co-occur with FAS, stemming from prenatal alcohol exposure. However, these conditions are considered alcohol-related birth defects[16] and not diagnostic criteria for FAS.
- Heart: A heart murmur that frequently disappears by one year of age. Ventricular septal defect most commonly seen, followed by an atrial septal defect.
- Bones: Joint anomalies including abnormal position and function, altered palmar crease patterns, small distal phalanges, and small fifth fingernails.
- Kidneys: Horseshoe, aplastic, dysplastic, or hypoplastic kidneys.
- Eyes: Strabismus, optic nerve hypoplasia[36] (which may cause light sensitivity, decreased visual acuity, or involuntary eye movements).
- Occasional problems: ptosis of the eyelid, microphthalmia, cleft lip with or without a cleft palate, webbed neck, short neck, tetralogy of Fallot, coarctation of the aorta, spina bifida, and hydrocephalus.
Cause
Fetal alcohol syndrome usually occurs when a pregnant woman has more than four standard drinks per day.[37] Milder symptoms have been found with two drinks per day during the early part of pregnancy.[37][38] Among those who are alcoholic, about a third of children have FAS.[37]
Evidence of harm from less than two drinks per day or 10 drinks per week is not clear.[37][39] While small amounts of alcohol do not cause an abnormal appearance, it may cause behavioral issues.[11] There is conflicting evidence regarding whether drinking by fathers before conception can cause FAS.[37]
Mechanism
Despite intense research efforts, the exact mechanism for the development of FAS or FASD is unknown. On the contrary, clinical and animal studies have identified a broad spectrum of pathways through which maternal alcohol can negatively affect the outcome of a pregnancy. Clear conclusions with universal validity are difficult to draw, since different ethnic groups show considerable genetic polymorphism for the hepatic enzymes responsible for ethanol detoxification.[40]
Genetic examinations have revealed a continuum of long-lasting molecular effects that are not only timing specific but are also dosage specific; with even moderate amounts being able to cause alterations.[41]
A human fetus appears to be at triple risk from maternal alcohol consumption:[42][43]
- The placenta allows free entry of ethanol and toxic metabolites like acetaldehyde into the fetal compartment. The so-called placental barrier is practically absent with respect to ethanol.
- The developing fetal nervous system appears particularly sensitive to ethanol toxicity. The latter interferes with proliferation, differentiation, neuronal migration, axonic outgrowth, integration, and fine-tuning of the synaptic network. In short, all major processes in the developing central nervous system appear compromised.
- Fetal tissues are quite different from adult tissues in function and purpose. For example, the main detoxicating organ in adults is the liver, whereas the fetal liver is incapable of detoxifying ethanol, as the ADH and ALDH enzymes have not yet been brought to expression at this early stage. Up to term, fetal tissues do not have significant capacity for the detoxification of ethanol, and the fetus remains exposed to ethanol in the amniotic fluid for periods far longer than the decay time of ethanol in the maternal circulation. The lack of significant quantities of ADH and ALDH means that fetal tissues have much lower quantities of antioxidant enzymes, like SOD, glutathione transferases, and glutathion peroxidases, resulting in antioxidant protection being much less effective.
Diagnosis
Because admission of alcohol use during pregnancy can stigmatize birth mothers, many are reluctant to admit drinking or to provide an accurate report of the quantity they drank. This complicates diagnosis and treatment [23] of the syndrome. As a result, diagnosis of the severity of FASD relies on protocols of observation of the child's physiology and behavior rather than maternal self-reporting. Presently, four FASD diagnostic systems that diagnose FAS and other FASD conditions have been developed in North America:
- The Institute of Medicine's guidelines for FAS, the first system to standardize diagnoses of individuals with prenatal alcohol exposure;[16]
- The University of Washington's "The 4-Digit Diagnostic Code", which ranks the four key features of FASD on a Likert scale of one to four and yields 256 descriptive codes that can be categorized into 22 distinct clinical categories, ranging from FAS to no findings;[20]
- The Centers for Disease Control's "Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis", which established consensus on the diagnosis FAS in the U.S. but deferred addressing other FASD conditions;[23] and
- Canadian guidelines for FASD diagnoses, which established criteria for diagnosing FASD in Canada and harmonized most differences between the IOM and University of Washington's systems.[2]
Each diagnostic system requires that a complete FASD evaluation includes an assessment of the four key features of FASD, described below. A positive finding on all four features is required for a diagnosis of FAS. However, prenatal alcohol exposure and central nervous system damage are the critical elements of the spectrum of FASD, and a positive finding in these two features is sufficient for an FASD diagnosis that is not "full-blown FAS".
While the four diagnostic systems essentially agree on criteria for fetal alcohol syndrome (FAS), there are still differences when full criteria for FAS are not met. This has resulted in differing and evolving nomenclature for other conditions across the spectrum of FASD, which may account for such a wide variety of terminology. Most individuals with deficits resulting from prenatal alcohol exposure do not express all features of FAS and fall into other FASD conditions.[16] The Canadian guidelines recommend the assessment and descriptive approach of the "4-Digit Diagnostic Code" for each key feature of FASD and the terminology of the IOM in diagnostic categories, excepting ARBD.[2]
Thus, other FASD conditions are partial expressions of FAS. However, these other FASD conditions may create disabilities similar to FAS if the key area of central nervous system damage shows clinical deficits in two or more of ten domains of brain functioning. Essentially, even though growth deficiency and/or FAS facial features may be mild or nonexistent in other FASD conditions, yet clinically significant brain damage of the central nervous system is present. In these other FASD conditions, an individual may be at greater risk for adverse outcomes because brain damage is present without associated visual cues of poor growth or the "FAS face" that might ordinarily trigger an FASD evaluation. Such individuals may be misdiagnosed with primary mental health disorders such as ADHD or oppositional defiance disorder without appreciation that brain damage is the underlying cause of these disorders, which requires a different treatment paradigm than typical mental health disorders. While other FASD conditions may not yet be included as an ICD or DSM-IV-TR diagnosis, they nonetheless pose significant impairment in functional behavior because of underlying brain damage.
Fetal alcohol syndrome
The following criteria must be fully met for an FAS diagnosis:[2][16][20][23]
- Growth deficiency: Prenatal or postnatal height or weight (or both) at or below the 10th percentile[21]
- FAS facial features: All three FAS facial features present[29]
- Central nervous system damage: Clinically significant structural neurological, or functional impairment
- Prenatal alcohol exposure: Confirmed or Unknown prenatal alcohol exposure
Fetal alcohol syndrome (FAS) is the first diagnosable condition of FASD that was discovered. FAS is the only expression of FASD that has garnered consensus among experts to become an official ICD-9 and ICD-10 diagnosis. To make this diagnosis or determine any FASD condition, a multi-disciplinary evaluation is necessary to assess each of the four key features for assessment. Generally, a trained physician will determine growth deficiency and FAS facial features. While a qualified physician may also assess central nervous system structural abnormalities and/or neurological problems, usually central nervous system damage is determined through psychological, speech-language, and occupational therapy assessments to ascertain clinically significant impairments in three or more of the Ten Brain Domains.[44] Prenatal alcohol exposure risk may be assessed by a qualified physician, psychologist, social worker, or chemical health counselor. These professionals work together as a team to assess and interpret data of each key feature for assessment and develop an integrative, multi-disciplinary report to diagnose FAS (or other FASD conditions) in an individual.
Partial FAS
Partial FAS (pFAS) was previously known as atypical FAS in the 1997 edition of the "4-Digit Diagnostic Code". People with pFAS have a confirmed history of prenatal alcohol exposure, but may lack growth deficiency or the complete facial stigmata. Central nervous system damage is present at the same level as FAS. These individuals have the same functional disabilities but "look" less like FAS.
The following criteria must be fully met for a diagnosis of Partial FAS:[2][16][20]
- Growth deficiency: Growth or height may range from normal to deficient[21]
- FAS facial features: Two or three FAS facial features present[29]
- Central nervous system damage: Clinically significant structural, neurological, or functional impairment in three or more of the Ten Brain Domains[44]
- Prenatal alcohol exposure: Confirmed prenatal alcohol exposure
Fetal alcohol effects
Fetal alcohol effects (FAE) is a previous term for alcohol-related neurodevelopmental disorder and alcohol-related birth defects.[1] It was initially used in research studies to describe humans and animals in whom teratogenic effects were seen after confirmed prenatal alcohol exposure (or unknown exposure for humans), but without obvious physical anomalies.[45] Smith (1981) described FAE as an "extremely important concept" to highlight the debilitating effects of brain damage, regardless of the growth or facial features.[46] This term has fallen out of favor with clinicians because it was often regarded by the public as a less severe disability than FAS, when in fact its effects can be just as detrimental.[47]
Alcohol-related neurodevelopmental disorder
Alcohol-related neurodevelopmental disorder (ARND) was initially suggested by the Institute of Medicine to replace the term FAE and focus on central nervous system damage, rather than growth deficiency or FAS facial features. The Canadian guidelines also use this diagnosis and the same criteria. While the "4-Digit Diagnostic Code" includes these criteria for three of its diagnostic categories, it refers to this condition as static encephalopathy. The behavioral effects of ARND are not necessarily unique to alcohol however, so use of the term must be within the context of confirmed prenatal alcohol exposure.[48] ARND may be gaining acceptance over the terms FAE and ARBD to describe FASD conditions with central nervous system abnormalities or behavioral or cognitive abnormalities or both due to prenatal alcohol exposure without regard to growth deficiency or FAS facial features.[48][49]
The following criteria must be fully met for a diagnosis of ARND or static encephalopathy:[2][16][20]
- Growth deficiency: Growth or height may range from normal to minimally deficient[21]
- FAS facial features: Minimal or no FAS facial features present[29]
- Central nervous system damage: Clinically significant structural, neurological, or functional impairment in three or more of the Ten Brain Domains[44]
- Prenatal alcohol exposure: Confirmed prenatal alcohol exposure;0
Alcohol-related birth defects
Alcohol-related birth defects (ARBD), formerly known as possible fetal alcohol effect (PFAE),[45] was a term proposed as an alternative to FAE and PFAE[50] The IOM presents ARBD as a list of congenital anomalies that are linked to maternal alcohol use but have no key features of FASD.[16] PFAE and ARBD have fallen out of favor because these anomalies are not necessarily specific to maternal alcohol consumption and are not criteria for diagnosis of FASD.[48] The Canadian guidelines recommend that ARBD should not be used as an umbrella term or diagnostic category for FASD.
Exposure
Prenatal alcohol exposure is determined by interview of the biological mother or other family members knowledgeable of the mother's alcohol use during the pregnancy (if available), prenatal health records (if available), and review of available birth records, court records (if applicable), chemical dependency treatment records (if applicable), chemical biomarkers,[51] or other reliable sources.
Exposure level is assessed as confirmed exposure, unknown exposure, and confirmed absence of exposure by the IOM, CDC and Canadian diagnostic systems. The "4-Digit Diagnostic Code" further distinguishes confirmed exposure as High Risk and Some Risk:
- High Risk: Confirmed use of alcohol during pregnancy known to be at high blood alcohol levels (100 mg/dL or greater) delivered at least weekly in early pregnancy.
- Some Risk: Confirmed use of alcohol during pregnancy with use less than High Risk or unknown usage patterns.
- Unknown Risk: Unknown use of alcohol during pregnancy.
- No Risk: Confirmed absence of prenatal alcohol exposure.
Confirmed exposure
Amount, frequency, and timing of prenatal alcohol use can dramatically impact the other three key features of FASD. While consensus exists that alcohol is a teratogen, there is no clear consensus as to what level of exposure is toxic.[16] The CDC guidelines are silent on these elements diagnostically. The IOM and Canadian guidelines explore this further, acknowledging the importance of significant alcohol exposure from regular or heavy episodic alcohol consumption in determining, but offer no standard for diagnosis. Canadian guidelines discuss this lack of clarity and parenthetically point out that "heavy alcohol use" is defined by the National Institute on Alcohol Abuse and Alcoholism as five or more drinks per episode on five or more days during a 30-day period.[52]
"The 4-Digit Diagnostic Code" ranking system distinguishes between levels of prenatal alcohol exposure as high risk and some risk. It operationalizes high risk exposure as a blood alcohol concentration (BAC) greater than 100 mg/dL delivered at least weekly in early pregnancy. This BAC level is typically reached by a 55 kg female drinking six to eight beers in one sitting.[20]
Unknown exposure
For many adopted or adults and children in foster care, records or other reliable sources may not be available for review. Reporting alcohol use during pregnancy can also be stigmatizing to birth mothers, especially if alcohol use is ongoing.[23] In these cases, all diagnostic systems use an unknown prenatal alcohol exposure designation. A diagnosis of FAS is still possible with an unknown exposure level if other key features of FASD are present at clinical levels.
Confirmed absence of exposure
Confirmed absence of exposure would apply to planned pregnancies in which no alcohol was used or pregnancies of women who do not use alcohol or report no use during the pregnancy. This designation is relatively rare, as most people presenting for an FASD evaluation are at least suspected to have had a prenatal alcohol exposure due to presence of other key features of FASD.[20][23]
Biomarkers
Evidence is insufficient for the use of chemical biomarkers to detect prenatal alcohol exposure.[53] Biomarkers being studied include fatty acid ethyl esters (FAEE) detected in the meconium (first feces of an infant) and hair. FAEE may be present if chronic alcohol exposure occurs during the 2nd and 3rd trimester since this is when the meconium begins to form. Concentrations of FAEE can be influence by medication use, diet, and individual genetic variations in FAEE metabolism however.[54][55]
Ten brain domains
A recent effort to standardize assessment of functional CNS damage has been suggested by an experienced FASD diagnostic team in Minnesota. The proposed framework attempts to harmonize IOM, 4-Digit Diagnostic Code, CDC, and Canadian guidelines for measuring CNS damage vis-à-vis FASD evaluations and diagnosis. The standardized approach is referred to as the Ten Brain Domains and encompasses aspects of all four diagnostic systems' recommendations for assessing CNS damage due to prenatal alcohol exposure. The framework provides clear definitions of brain dysfunction, specifies empirical data needed for accurate diagnosis, and defines intervention considerations that address the complex nature of FASD with the intention to avoid common secondary disabilities.[44]
The proposed Ten Brain Domains include:[44]
- Achievement, adaptive behavior, attention, cognition, executive functioning, language, memory, motor skills, multisensory integration or soft neurological problems, social communication[44]
The Fetal Alcohol Diagnostic Program (FADP) uses unpublished Minnesota state criteria of performance at 1.5 or more standard deviations on standardized testing in three or more of the Ten Brain Domains to determine CNS damage. However, the Ten Brain Domains are easily incorporated into any of the four diagnostic systems' CNS damage criteria, as the framework only proposes the domains, rather than the cut-off criteria for FASD.[56]
Differential diagnosis
The CDC reviewed nine syndromes that have overlapping features with FAS; however, none of these syndromes include all three FAS facial features, and none are the result of prenatal alcohol exposure:[23]
- Aarskog syndrome
- Williams syndrome
- Noonan syndrome
- Dubowitz syndrome
- Brachman-DeLange syndrome
- Toluene syndrome
- Fetal hydantoin syndrome
- Fetal valproate syndrome
- Maternal PKU fetal effects
Other disorders that have similar symptoms may include:[57]
Prevention
The only certain way to prevent FAS is to avoid drinking alcohol during pregnancy.[48][58] In the United States, the Surgeon General recommended in 1981, and again in 2005, that women abstain from alcohol use while pregnant or while planning a pregnancy, the latter to avoid damage even in the earliest stages (even weeks) of a pregnancy, as the woman may not be aware that she has conceived.[15] The Center for Disease Control and the American College of Obstetricians and Gynecologists also recommend no alcohol during pregnancy.[55] In the United States, federal legislation has required that warning labels be placed on all alcoholic beverage containers since 1988 under the Alcoholic Beverage Labeling Act.
There is some controversy surrounding the "zero-tolerance" approach taken by many countries when it comes to alcohol consumption during pregnancy. The assertion that moderate drinking causes FAS is said to lack strong evidence and, in fact, the practice of equating a responsible level of drinking with potential harm to the fetus may have negative social, legal, and health impacts.[59] In addition, special care should be taken when considering statistics on this disease, as prevalence and causation is often linked with FASD, which is more common and causes less harm, as opposed to FAS.[60]
Treatment
There is no cure for FASD, but treatment is possible. Early intervention from birth to age 3 has been shown to improve the development of a child born with FASD.[55] Because CNS damage, symptoms, secondary disabilities, and needs vary widely by individual, there is no one treatment type that works for everyone.
Medication
Psychoactive drugs are frequently tried on those with FASD as many FASD symptoms are mistaken for or overlap with other disorders, most notably ADHD.[61]
Behavioral interventions
Behavioral interventions are based on the learning theory, which is the basis for many parenting and professional strategies and interventions.[49] Along with ordinary parenting styles, such strategies are frequently used by default for treating those with FAS, as the diagnoses oppositional defiance disorder (ODD), conduct disorder, reactive attachment disorder (RAD) often overlap with FAS (along with ADHD), and these are sometimes thought to benefit from behavioral interventions. Frequently, a person's poor academic achievement results in special education services, which also utilizes principles of learning theory, behavior modification, and outcome-based education.
Developmental framework
Many books and handouts on FAS recommend a developmental approach, based on developmental psychology, even though most do not specify it as such and provide little theoretical background. Optimal human development generally occurs in identifiable stages (e.g., Jean Piaget's theory of cognitive development, Erik Erikson's stages of psychosocial development, John Bowlby's attachment framework, and other developmental stage theories). FAS interferes with normal development,[62] which may cause stages to be delayed, skipped, or immaturely developed. Over time, an unaffected child can negotiate the increasing demands of life by progressing through stages of development normally, but not so for a child with FAS.[62]
By knowing what developmental stages and tasks children follow, treatment and interventions for FAS can be tailored to helping a person meet developmental tasks and demands successfully.[62] If a person is delayed in the adaptive behavior domain, for instance, then interventions would be recommended to target specific delays through additional education and practice (e.g., practiced instruction on tying shoelaces), giving reminders, or making accommodations (e.g., using slip-on shoes) to support the desired functioning level. This approach is an advance over behavioral interventions, because it takes the person's developmental context into account while developing interventions.
Advocacy model
The advocacy model takes the point of view that someone is needed to actively mediate between the environment and the person with FAS.[48] Advocacy activities are conducted by an advocate (for example, a family member, friend, or case manager) and fall into three basic categories. An advocate for FAS: (1) interprets FAS and the disabilities that arise from it and explains it to the environment in which the person operates, (2) engenders change or accommodation on behalf of the person, and (3) assists the person in developing and reaching attainable goals.[48]
The advocacy model is often recommended, for example, when developing an Individualized Education Program (IEP) for the person's progress at school.[61]
An understanding of the developmental framework would presumably inform and enhance the advocacy model, but advocacy also implies interventions at a systems level as well, such as educating schools, social workers, and so forth on best practices for FAS. However, several organizations devoted to FAS also use the advocacy model at a community practice level as well.[63]
Public health and policy
Treating FAS at the public health and public policy level promotes FAS prevention and diversion of public resources to assist those with FAS.[48] It is related to the advocacy model but promoted at a systems level (rather than with the individual or family), such as developing community education and supports, state or province level prevention efforts (e.g., screening for maternal alcohol use during OB/GYN or prenatal medical care visits), or national awareness programs. Several organizations and state agencies in the U.S. are dedicated to this type of intervention.[63]
The US Centers for Disease Control estimates 3 million women in the United States are at risk of having a baby with FASD, and recommended that women of child-bearing age should be on birth control or abstain from drinking alcohol as the safest way to avoid this.[64]
Prognosis
Primary disabilities
The primary disabilities of FAS are the functional difficulties with which the child is born as a result of CNS damage due to prenatal alcohol exposure.[65]
Often, primary disabilities are mistaken as behavior problems, but the underlying CNS damage is the originating source of a functional difficulty,[66] rather than a mental health condition, which is considered a secondary disability. The exact mechanisms for functional problems of primary disabilities are not always fully understood, but animal studies have begun to shed light on some correlates between functional problems and brain structures damaged by prenatal alcohol exposure.[48] Representative examples include:
- Learning impairments are associated with impaired dendrites of the hippocampus[67]
- Impaired motor development and functioning are associated with reduced size of the cerebellum[68]
- Hyperactivity is associated with decreased size of the corpus callosum[69]
Functional difficulties may result from CNS damage in more than one domain, but common functional difficulties by domain include:[48][49][62][66] Note that this is not an exhaustive list of difficulties.
- Achievement: Learning disabilities
- Adaptive behavior: Poor impulse control, poor personal boundaries, poor anger management, stubbornness, intrusive behavior, too friendly with strangers, poor daily living skills, developmental delays
- Attention: Attention-Deficit/Hyperactivity Disorder (ADHD), poor attention or concentration, distractible
- Cognition: Intellectual disability, confusion under pressure, poor abstract skills, difficulty distinguishing between fantasy and reality, slower cognitive processing
- Executive functioning: Poor judgment, Information-processing disorder, poor at perceiving patterns, poor cause and effect reasoning, inconsistent at linking words to actions, poor generalization ability
- Language: Expressive or receptive language disorders, grasp parts but not whole concepts, lack understanding of metaphor, idioms, or sarcasm
- Memory: Poor short-term memory, inconsistent memory and knowledge base
- Motor skills: Poor handwriting, poor fine motor skills, poor gross motor skills, delayed motor skill development (e.g., riding a bicycle at appropriate age)
- Sensory processing and soft neurological problems: sensory processing disorder, sensory defensiveness, undersensitivity to stimulation
- Social communication: Intrude into conversations, inability to read nonverbal or social cues, "chatty" but without substance
Secondary disabilities
The secondary disabilities of FAS are those that arise later in life secondary to CNS damage. These disabilities often emerge over time due to a mismatch between the primary disabilities and environmental expectations; secondary disabilities can be ameliorated with early interventions and appropriate supportive services.[65]
Six main secondary disabilities were identified in a University of Washington research study of 473 subjects diagnosed with FAS, PFAS (partial fetal alcohol syndrome), and ARND (alcohol-related neurodevelopmental disorder):[48][65]
- Mental health problems: Diagnosed with ADHD, Clinical Depression, or other mental illness, experienced by over 90% of the subjects
- Disrupted school experience: Suspended or expelled from school or dropped out of school, experienced by 60% of the subjects (age 12 and older)
- Trouble with the law: Charged or convicted with a crime, experienced by 60% of the subjects (age 12 and older)
- Confinement: For inpatient psychiatric care, inpatient chemical dependency care, or incarcerated for a crime, experienced by about 50% of the subjects (age 12 and older)
- Inappropriate sexual behavior: Sexual advances, sexual touching, or promiscuity, experienced by about 50% of the subjects (age 12 and older)
- Alcohol and drug problems: Abuse or dependency, experienced by 35% of the subjects (age 12 and older)
Two additional secondary disabilities exist for adults:[48][65]
- Dependent living: Group home, living with family or friends, or some sort of assisted living, experienced by 80% of the subjects (age 21 and older)
- Problems with employment: Required ongoing job training or coaching, could not keep a job, unemployed, experienced by 80% of the subjects (age 21 and older)
Protective factors and strengths
Eight factors were identified in the same study as universal protective factors that reduced the incidence rate of the secondary disabilities:[48][65]
- Living in a stable and nurturing home for over 73% of life
- Being diagnosed with FAS before age six
- Never having experienced violence
- Remaining in each living situation for at least 2.8 years
- Experiencing a "good quality home" (meeting 10 or more defined qualities) from age 8 to 12 years old
- Having been found eligible for developmental disability (DD) services
- Having basic needs met for at least 13% of life
- Having a diagnosis of FAS (rather than another FASD condition)
Malbin (2002) has identified the following areas of interests and talents as strengths that often stand out for those with FASD and should be utilized, like any strength, in treatment planning:[49]
- Music, playing instruments, composing, singing, art, spelling, reading, computers, mechanics, woodworking, skilled vocations (welding, electrician, etc.), writing, poetry
- Participation in non-impact sport or physical fitness activities
Epidemiology
FASD is estimated to affect between 2% and 5% of people in the United States and Western Europe.[6] FAS is believed to occur in between 0.2 and 9 per 1000 live births in the United States.[6] The lifetime costs of an individual with FAS were estimated to be two million USD in 2002.[6] Drinking any quantity during pregnancy, the risk of giving birth to a child with FASD is about 15%, and to a child with FAS about 1.5%. Drinking large quantities, defined as 2 standard drinks a day, or 6 standard drinks in a short time, carries a 50% risk of a FAS birth.[70]
Australia
FASD among Australian youth is more common in indigenous Australians.[71] The only states that have registered birth defects in Australian youth are Western Australia, New South Wales, Victoria and South Australia.[72] In Australia, only 12% of Australian health professionals are aware of the diagnostics and symptoms of FASD.[71] In Western Australia, the rate of births resulting in FASD is 0.02 per 1,000 births for non-Indigenous Australians, however among indigenous births the rate is 2.76 per 1,000 births.[72] In Victoria, there have been no registered FASD related births for indigenous Australians, but the rate for the general population in Victoria is 0.01–0.03 per 1000 births.[72] There have been no dedicated FASD clinics within Western Australia, but there are also no nationally supported diagnostic criteria anywhere in Australia.[73] Passive surveillance is a prevention technique used within Australia to assist in monitoring and establishing detectable defects during pregnancy and childhood.[72]
History
From the 1960s to the 1980s, alcohol was commonly used as a tocolytic, a method to stop preterm labor. The method originated with Dr. Fritz Fuchs, the chairman of the department of obstetrics and gynecology at Cornell University Medical College.[74][75] Doctors recommended a small amount of alcohol to calm the uterus during contractions in early pregnancy or Braxton Hicks contractions. In later stages of pregnancy, the alcohol was administered intravenously and often in large amounts. "Women experienced similar effects as occur with oral ingestion, including intoxication, nausea and vomiting, and potential alcohol poisoning, followed by hangovers when the alcohol was discontinued."[76] Vomiting put the mother at a high risk for aspiration and was "a brutal procedure for all involved."[74] Because the alcohol was being given intravenously, the doctor could continue giving the treatment to the mother long after she had passed out, resulting in her being more intoxicated than would otherwise be possible. Such heavy intoxication is highly likely to contribute to FASD.[74]
Historical references
Anecdotal accounts of prohibitions against maternal alcohol use from Biblical, ancient Greek, and ancient Roman sources[77] imply a historical awareness of links between maternal alcohol use and negative child outcomes.[34] For example, in the Bible, Judges 13:4 (addressed to a woman who was going to have a baby) reads: "Therefore be careful and drink no wine or strong drink, and eat nothing unclean" (ESV). In 1725 British physicians petitioned the House of Commons on the effects of strong drink when consumed by pregnant women saying that such drinking is “… too often the cause of weak, feeble, and distempered children, who must be, instead of an advantage and strength, a charge to their country.”[78] There are many other such historical references. In Gaelic Scotland, the mother and nurse were not allowed to consume ale during pregnancy and breastfeeding (Martin Martin). Claims that alcohol consumption caused idiocy were part of the Teetotalism's message in the 19th century,[79] but such claims, despite some attempts to offer evidence, were ignored because no mechanism could be advanced.[80]
The earliest recorded observation of possible links between maternal alcohol use and fetal damage was made in 1899 by Dr. William Sullivan, a Liverpool prison physician who noted higher rates of stillbirth for 120 alcoholic female prisoners than their sober female relatives; he suggested the causal agent to be alcohol use.[81] This contradicted the predominating belief at the time that heredity caused intellectual disability, poverty, and criminal behavior, which contemporary studies on the subjects usually concluded.[48] A case study by Henry H. Goddard of the Kallikak family—popular in the early 1900s—represents this earlier perspective,[82] though later researchers have suggested that the Kallikaks almost certainly had FAS.[83] General studies and discussions on alcoholism throughout the mid-1900s were typically based on a heredity argument.[84]
Prior to fetal alcohol syndrome being specifically identified and named in 1973, only a few studies had noted differences between the children of mothers who used alcohol during pregnancy or breast-feeding and those who did not, and identified alcohol use as a possible contributing factor rather than heredity.[48]
Recognition as a syndrome
Fetal alcohol syndrome was named in 1973 by two dysmorphologists, Drs. Kenneth Lyons Jones and David Weyhe Smith of the University of Washington Medical School in Seattle, United States. They identified a pattern of "craniofacial, limb, and cardiovascular defects associated with prenatal onset growth deficiency and developmental delay" in eight unrelated children of three ethnic groups, all born to mothers who were alcoholics.[85] The pattern of malformations indicated that the damage was prenatal. News of the discovery shocked some, while others were skeptical of the findings.[86]
Dr. Paul Lemoine of Nantes, France had already published a study in a French medical journal in 1968 about children with distinctive features whose mothers were alcoholics,[87] and in the U.S., Christy Ulleland and colleagues at the University of Washington Medical School had conducted an 18-month study in 1968–1969 documenting the risk of maternal alcohol consumption among the offspring of 11 alcoholic mothers.[88] The Washington and Nantes findings were confirmed by a research group in Gothenburg, Sweden in 1979.[89] Researchers in France, Sweden, and the United States were struck by how similar these children looked, though they were not related, and how they behaved in the same unfocused and hyperactive manner.[89]
Within nine years of the Washington discovery, animal studies, including non-human monkey studies carried out at the University of Washington Primate Center by Dr. Sterling Clarren, had confirmed that alcohol was a teratogen. By 1978, 245 cases of FAS had been reported by medical researchers, and the syndrome began to be described as the most frequent known cause of intellectual disability.
While many syndromes are eponymous, i.e. named after the physician first reporting the association of symptoms, Dr. Smith named FAS after the causal agent of the symptoms.[90] He reasoned that doing so would encourage prevention, believing that if people knew maternal alcohol consumption caused the syndrome, then abstinence during pregnancy would follow from patient education and public awareness.[90] At the time, nobody was aware of the full range of possible birth defects from FAS or its rate of prevalence.[90] Over time, as subsequent research and clinical experience suggested that a range of effects (including physical, behavioral, and cognitive) could arise from prenatal alcohol exposure, the term Fetal Alcohol Spectrum Disorder (FASD) was developed to include FAS as well as other conditions resulting from prenatal alcohol exposure.[90] Currently, FAS[16][45][85] is the only expression of prenatal alcohol exposure defined by the International Statistical Classification of Diseases and Related Health Problems and assigned ICD-9 and diagnoses.
In fiction
In Aldous Huxley's 1932 novel Brave New World (where all fetuses are gestated in vitro in a factory) lower caste fetuses receive alcohol transfusions on purpose to reduce intelligence and height and thus condition them for simple menial tasks. It is noteworthy that Huxley's description of the more obvious effects predates official medical recognition (1973) by more than four decades but are most likely inspired by the centuries-old inofficial claims.
See also
- Alcohol and pregnancy
- Smoking and pregnancy
- Environmental toxicants and fetal development
References
- "Facts about FASDs". 16 April 2015. Archived from the original on 23 May 2015. Retrieved 10 June 2015.
- Chudley; et al. (2005). "Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis". CMAJ. 172 (5 Suppl): S1–S21. doi:10.1503/cmaj.1040302. PMC 557121. PMID 15738468.
- Rasmussen, Carmen; Andrew, Gail; Zwaigenbaum, Lonnie; Tough, Suzanne (20 November 2016). "Neurobehavioural outcomes of children with fetal alcohol spectrum disorders: A Canadian perspective". Paediatrics & Child Health. 13 (3): 185–191. ISSN 1205-7088. PMC 2529423. PMID 19252695.
- "Alcohol Use in Pregnancy". 17 April 2014. Archived from the original on 28 June 2015. Retrieved 10 June 2015.
- Roszel, EL (13 April 2015). "Central nervous system deficits in fetal alcohol spectrum disorder". The Nurse Practitioner. 40 (4): 24–33. doi:10.1097/01.npr.0000444650.10142.4f. PMID 25774812.
- "Data & Statistics Prevalence of FASDs". Center for Disease Control and Prevention. 16 April 2015. Archived from the original on 29 June 2015. Retrieved 10 June 2015.
- Coriale, G; Fiorentino, D; Di Lauro, F; Marchitelli, R; Scalese, B; Fiore, M; Maviglia, M; Ceccanti, M (2013). "Fetal Alcohol Spectrum Disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment". Rivista di Psichiatria. 48 (5): 359–69. doi:10.1708/1356.15062. PMID 24326748.
- Riley, EP; Infante, MA; Warren, KR (June 2011). "Fetal alcohol spectrum disorders: an overview". Neuropsychology Review. 21 (2): 73–80. doi:10.1007/s11065-011-9166-x. PMC 3779274. PMID 21499711.
- "Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Spectrum Disorders (FASD) – conditions and interventions". www.sbu.se. Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). 14 December 2016. Archived from the original on 6 June 2017. Retrieved 8 June 2017.
- "Fetal Alcohol Exposure". April 2015. Archived from the original on 10 June 2015. Retrieved 10 June 2015.
- McHugh, RK; Wigderson, S; Greenfield, SF (June 2014). "Epidemiology of substance use in reproductive-age women". Obstetrics and Gynecology Clinics of North America. 41 (2): 177–89. doi:10.1016/j.ogc.2014.02.001. PMC 4068964. PMID 24845483.
- Gupta, Keshav Kumar; Gupta, Vinay Kumar; Shirasaka, Tomohiro (2016). "An Update on Fetal Alcohol Syndrome—Pathogenesis, Risks, and Treatment". Alcoholism: Clinical and Experimental Research. 40 (8): 1594–1602. doi:10.1111/acer.13135. PMID 27375266.
- Williams, J. F.; Smith, V. C. (19 October 2015). "Fetal Alcohol Spectrum Disorders". Pediatrics. 136 (5): e1395–e1406. doi:10.1542/peds.2015-3113. PMID 26482673.
- Fetal Alcohol Spectrum Disorder: Management and Policy Perspectives of FASD. John Wiley & Sons. 2011. pp. 73–75. ISBN 9783527632565. Archived from the original on 10 September 2017.
- Vice Admiral Richard H. Carmona (2005). "A 2005 Message to Women from the U.S. Surgeon General" (PDF). Archived (PDF) from the original on 24 September 2015. Retrieved 12 June 2015.
- Institute of Medicine; Committee to Study Fetal Alcohol Syndrome (1995). Stratton, Kathleen; Howe, Cynthia; Battaglia, Frederick C. (eds.). Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington, D.C.: National Academy Press. ISBN 978-0-309-05292-4. Archived from the original on 11 March 2016.
- "Australian Government National Health and Medical Research Council". Archived from the original on 5 November 2012. Retrieved 4 November 2012.
- Kingdon; et al. (2016), "Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder – a meta-analysis", Journal of Child Psychology and Psychiatry, 57 (2): 116–131, doi:10.1111/jcpp.12451, PMC 5760222, PMID 26251262
- "CAMH: More than 400 conditions co-occur with Fetal Alcohol Spectrum Disorders (FASD), CAMH study finds". www.camh.ca. Archived from the original on 21 November 2016. Retrieved 20 November 2016.
- Astley, S.J. (2004). Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. Seattle: University of Washington. PDF available at FAS Diagnostic and Prevention Network Archived 16 December 2006 at the Wayback Machine. Retrieved on 2007-04-11.
- Clinical growth charts. Archived 3 December 2010 at the Wayback Machine National Center for Growth Statistics. Retrieved on 2007-04-10
- del Campo, Miguel; Jones, Kenneth Lyons (1 January 2017). "A review of the physical features of the fetal alcohol spectrum disorders". European Journal of Medical Genetics. Special issue on Environmental Teratogens. 60 (1): 55–64. doi:10.1016/j.ejmg.2016.10.004. ISSN 1769-7212. PMID 27729236.
- Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis (PDF). CDC (July 2004). Retrieved on 2019-10-19
- Jones K, Smith D (1975). "The fetal alcohol syndrome". Teratology. 12 (1): 1–10. doi:10.1002/tera.1420120102. PMID 1162620.
- Renwick J, Asker R (1983). "Ethanol-sensitive times for the human conceptus". Early Hum Dev. 8 (2): 99–111. doi:10.1016/0378-3782(83)90065-8. PMID 6884260.
- Astley SJ, Clarren SK (1996). "Most FAS children have a smaller brain then other children "A case definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome"". Journal of Pediatrics. 129 (1): 33–41. doi:10.1016/s0022-3476(96)70187-7. PMID 8757560.
- Astley SJ, Stachowiak J, Clarren SK, Clausen C (2002). "Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population". Journal of Pediatrics. 141 (5): 712–717. doi:10.1067/mpd.2002.129030. PMID 12410204.
- Lip-philtrum guides Archived 8 February 2007 at the Wayback Machine. FAS Diagnostic and Prevention Network, University of Washington. Retrieved on 2007-04-10.
- FAS facial features Archived 27 October 2007 at the Wayback Machine. FAS Diagnostic and Prevention Network, University of Washington. Retrieved on 2007-04-10
- Astley, Susan. Backside of Lip-Philtrum Guides (2004) (PDF) Archived 19 June 2007 at the Wayback Machine. University of Washington, Fetal Alcohol Syndrome Diagnostic and Prevention Network. Retrieved on 2007-04-11
- West, J.R. (Ed.) (1986). Alcohol and Brain Development. New York: Oxford University Press.
- Clarren S, Alvord E, Sumi S, Streissguth A, Smith D (1978). "Brain malformations related to prenatal exposure to ethanol". J Pediatr. 92 (1): 64–7. doi:10.1016/S0022-3476(78)80072-9. PMID 619080.
- Coles C, Brown R, Smith I, Platzman K, Erickson S, Falek A (1991). "Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development". Neurotoxicol Teratol. 13 (4): 357–67. doi:10.1016/0892-0362(91)90084-A. PMID 1921915.
- Jones K.L.; Smith D.W. (1973). "Recognition of the fetal alcohol syndrome in early infancy". Lancet. 2 (7836): 999–1001. doi:10.1016/s0140-6736(73)91092-1. PMID 4127281.
- Mattson, S.N., & Riley, E.P. (2002). "Neurobehavioral and Neuroanatomical Effects of Heavy Prenatal Exposure to Alcohol," in Streissguth and Kantor. (2002). p. 10.
- Strömland K, Pinazo-Durán M (2002). "Ophthalmic involvement in the fetal alcohol syndrome: clinical and animal model studies". Alcohol Alcohol. 37 (1): 2–8. doi:10.1093/alcalc/37.1.2. PMID 11825849.
- Yaffe, Sumner J. (2011). Drugs in pregnancy and lactation : a reference guide to fetal and neonatal risk (9 ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 527. ISBN 9781608317080. Archived from the original on 10 September 2017.
- "Pregnancy and alcohol: occasional, light drinking may be safe". Prescrire Int. 21 (124): 44–50. February 2012. PMID 22413723.
- Henderson, J; Gray, R; Brocklehurst, P (March 2007). "Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome". BJOG : An International Journal of Obstetrics and Gynaecology. 114 (3): 243–52. doi:10.1111/j.1471-0528.2006.01163.x. PMID 17233797.
- Warren K.; Li T-K (2005). "Genetic polymorphisms: Impact on the risk of fetal alcohol spectrum disorders". Birth Defect Res A. 73 (4): 195–203. doi:10.1002/bdra.20125. PMID 15786496.
- Laufer BI, Mantha K, Kleiber ML, Diehl EJ, Addison SM, Singh SM (July 2013). "Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice". Disease Models & Mechanisms. 6 (4): 977–92. doi:10.1242/dmm.010975. PMC 3701217. PMID 23580197.
- Brien J.; et al. (1983). "Disposition of ethanol in human maternal venous blood and amniotic fluid". Am J Obstet Gynecol. 146 (2): 181–186. doi:10.1016/0002-9378(83)91050-5. PMID 6846436.
- Nava-Ocampo A.; et al. (2004). "Elimination kinetics of ethanol in pregnant women". Reproduct Toxicol. 18 (4): 613–617. doi:10.1016/j.reprotox.2004.02.012. PMID 15135856.
- Lang, Jeannette (2006). "Ten Brain Domains: A Proposal for Functional Central Nervous System Parameters for Fetal Alcohol Spectrum Disorder Diagnosis and Follow-up" (PDF). Journal of the FAS Institute. 4: 1–11. Archived from the original (PDF) on 26 September 2006. Retrieved 4 February 2007.
- Clarren S.K.; Smith D.W. (1978). "Fetal alcohol syndrome". New England Journal of Medicine. 298 (19): 1063–1067. doi:10.1056/NEJM197805112981906. PMID 347295.
- Smith D.W. (1981). "Fetal alcohol syndrome and fetal alcohol effects". Neurobehavioral Toxicology and Teratology. 3: 127.
- Aase J.M.; Jones K.L.; Clarren S.K. (1995). "Do we need the term FAE?". Pediatrics. 95 (3): 428–430. PMID 7862486.
- Streissguth, A. (1997). Fetal Alcohol Syndrome: A Guide for Families and Communities. Baltimore: Brookes Publishing. ISBN 1-55766-283-5.
- Malbin, D. (2002). Fetal Alcohol Spectrum Disorders: Trying Differently Rather Than Harder. Portland, Oregon: FASCETS, Inc. ISBN 0-9729532-0-5.
- Sokol, R. J.; Clarren, S. K. (1989). "Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring". Alcoholism: Clinical and Experimental Research. 13 (4): 597–598. doi:10.1111/j.1530-0277.1989.tb00384.x.
- Bager, Heidi; Christensen, Lars Porskjaer; Husby, Steffen; Bjerregaard, Lene (February 2017). "Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review". Alcoholism: Clinical and Experimental Research. 41 (2): 251–261. doi:10.1111/acer.13309.
- U.S. Department of Health and Human Services. (2000). National Institute on Alcohol Abuse and Alcoholism. Tenth special report to the U.S Congress on alcohol and health: Highlights frfom current research. Washington, DC: The Institute.
- McQuire, C.; Paranjothy, S.; Hurt, L.; Mann, M.; Farewell, D.; Kemp, A. (1 September 2016). "Objective Measures of Prenatal Alcohol Exposure: A Systematic Review". Pediatrics. 138 (3): e20160517. doi:10.1542/peds.2016-0517. ISSN 0031-4005. PMID 27577579.
- Bager, Heidi; Christensen, Lars Porskjær; Husby, Steffen; Bjerregaard, Lene (2017). "Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review". Alcoholism: Clinical and Experimental Research. 41 (2): 251–261. doi:10.1111/acer.13309. ISSN 1530-0277.
- Dejong, Katherine; Olyaei, Amy; Lo, Jamie O. (March 2019). "Alcohol Use in Pregnancy". Clinical Obstetrics and Gynecology. 62 (1): 142–155. doi:10.1097/GRF.0000000000000414. ISSN 0009-9201.
- FADP – Fetal Alcohol Diagnostic Program Archived 23 February 2007 at the Wayback Machine
- "Overlapping Behavioral Characteristics of FASD's & Related Mental Health Diagnosis". www.fasdfamilies.com. Cathy Bruer-Thompson. Retrieved 28 July 2019.
- "Alcohol and Pregnancy Questions and Answers | FASD | NCBDDD | CDC". www.cdc.gov. 4 August 2017. Retrieved 25 September 2017.
- Armstrong, E. M. (1 May 2000). "Fetal alcohol syndrome: the origins of a moral panic". Alcohol and Alcoholism. 35 (3): 276–282. doi:10.1093/alcalc/35.3.276.
- "Fetal Alcohol Spectrum Disorders (FASDs)". Center for Disease Control. Archived from the original on 28 July 2017. Retrieved 9 September 2017.
- Buxton, B. (2005). Damaged Angels: An Adoptive Mother Discovers the Tragic Toll of Alcohol in Pregnancy. New York: Carroll & Graf. ISBN 0-7867-1550-2.
- McCreight, B. (1997). Recognizing and Managing Children with Fetal Alcohol Syndrome/Fetal Alcohol Effects: A Guidebook. Washington, DC: CWLA. ISBN 0-87868-607-X.
- National Organization on Fetal Alcohol Syndrome, Archived 5 April 2007 at the Wayback Machine Minnesota Organization on Fetal Alcohol Syndrome. Archived 5 April 2007 at the Wayback Machine Retrieved on 2007-04-11
- "More than 3 million US women at risk for alcohol-exposed pregnancy | CDC Online Newsroom | CDC". www.cdc.gov. 2 February 2016. Archived from the original on 21 November 2016. Retrieved 20 November 2016.
- Streissguth, A.P., Barr, H.M., Kogan, J., & Bookstein, F.L. (1996). Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE): Final report to the Centers for Disease Control and Prevention on Grant No. RO4/CCR008515 (Tech. Report No. 96-06). Seattle: University of Washington, Fetal Alcohol and Drug Unit.
- Malbin, D. (1993). Fetal Alcohol Syndrome, Fetal Alcohol Effects: Strategies for Professionals. Center City, MN: Hazelden. ISBN 0-89486-951-5
- Abel EL, Jacobson S, Sherwin BT (1983). "In utero alcohol exposure: Functional and structural brain damage". Neurobehavioral Toxicology and Teratology. 5 (3): 363–366. PMID 6877477.
- Meyer L, Kotch L, Riley E (1990). "Neonatal ethanol exposure: functional alterations associated with cerebellar growth retardation". Neurotoxicol Teratol. 12 (1): 15–22. doi:10.1016/0892-0362(90)90107-N. PMID 2314357.
- Zimmerberg B, Mickus LA (1990). "Sex differences in corpus callosum: Influence of prenatal alcohol exposure and maternal undernutrition". Brain Research. 537 (1–2): 115–122. doi:10.1016/0006-8993(90)90347-e. PMID 2085766.
- Popova S.; Lange S.; Probst C.; Gmel G.; Rehm J. (2017). "Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis". The Lancet. 5: PE290–E299.
- Elliot, E. J; Payne, J; Hann, E; Bower, C (2006). "Diagnosis of fetal alcohol syndrome and alcohol use in pregnancy: A survey of Paediatricians' knowledge, attitudes and practice". Journal of Paediatrics and Child Health. 42 (1): 698–703. doi:10.1111/j.1440-1754.2006.00954.x. PMID 17044897.
- Burns, L; Breen, C; Bower, C; O'Leary, C; Elliiot, E. J (2015). "Counting fetal alcohol spectrum disorders in Australia:The evidence and the challenges". Drug and Alcohol Review. 32 (5): 461–467. doi:10.1111/dar.12047. PMID 23617437.
- Mutch, R. C; Watkins, R; Bower, C (2014). "Fetal alcohol spectrum disorders: Notifications to Western Australian register of developmental anomalies". Journal of Paediatrics and Child Health. 51 (4): 433–436. doi:10.1111/jpc.12746. PMID 25412883.
- Armstrong, E. M. (2003). Conceiving risk, bearing responsibility: Fetal alcohol syndrome & the diagnosis of moral disorder. Baltimore: The Johns Hopkins University Press.
- Saxon, Wolfgang (4 March 1995). "Dr. Fritz Fuchs, 76, Who Advanced Obstetrics". The New York Times. Archived from the original on 27 June 2017. Retrieved 16 February 2017.
- King, T. L., & Brucker, M. C. (2011). Pharmacology for women's health. Sudbury, MA: Jones and Bartlett.
- Oba, Peggy Seo (2007). "History of FASD". FAS Aware UK. Archived from the original on 4 January 2017. Retrieved 20 November 2016.
- Jackson, Randle (24 April 1828). Valpy, Abraham John (ed.). "Considerations on the Increase of Crime, and the Degree of its Extent". The Pamphleteer. London: John Hatchard and Son ... and T. and G. Underwood ... 29 (57): 325. OCLC 1761801.
[Alcohol consumption is] too often the cause of weak, feeble, and distempered children, who must be, instead of an advantage and strength, a charge to their country
. Biography of author Randle Jackson - Jonathan Townley Crane, Arts of Intoxication: The Aim and the Results. (New York:Carlton & Lanahan, 1870), 173–175
- Jennifer L. Tait The Poisoned Chalice (Tuscaloosa, AL: University of Alabama Press, 2010), 27,28.
- Sullivan W.C. (1899). "A note on the influence of maternal inebriety on the offspring". Journal of Mental Science. 45 (190): 489–503. doi:10.1192/bjp.45.190.489.
- Goddard, H.H. (1912). The Kallikak Family: A Study in the Heredity of Feeble-Mindedness. New York: Macmillan.
- Karp R.J.; Qazi Q.H.; Moller K.A.; Angelo W.A.; Davis J.M. (1995). "Fetal alcohol syndrome at the turn of the century: An unexpected explanation of the Kallikak family". Archives of Pediatrics and Adolescent Medicine. 149 (1): 45–48. doi:10.1001/archpedi.1995.02170130047010. PMID 7827659.
- Haggard, H.W., & Jellinek, E.M. (1942). Alcohol Explored. New York: Doubleday.
- Jones K.L.; Smith D.W; Ulleland C.N.; Streissguth A.P. (1973). "Pattern of malformation in offspring of chronic alcoholic mothers". Lancet. 1 (7815): 1267–1271. doi:10.1016/S0140-6736(73)91291-9. PMID 4126070.
- Streissguth, A.P. (2002). In A. Streissguth, & J. Kanter (Eds.), The Challenge in Fetal Alcohol Syndrome: Overcoming Secondary Disabilities. Seattle: University of WA Press. ISBN 0-295-97650-0.
- Lemoine P.; Harousseau H.; Borteyru J.B.; Menuet J.C. (1968). "Les enfants de parents alcooliques. Anomalies observées, à propos de 127 cas". Quest Medical. 21: 476–482. Reprinted in PMID 12657907
- Ulleland C.N. (1972). "The offspring of alcoholic mothers". Annals of the New York Academy of Sciences. 197 (1): 167–169. Bibcode:1972NYASA.197..167U. doi:10.1111/j.1749-6632.1972.tb28142.x. PMID 4504588.
- Olegard R.; Sabel K.G.; Aronsson M. Sandin; Johannsson P.R.; Carlsson C.; Kyllerman M.; Iversen K.; Hrbek A. (1979). "Effects on the child of alcohol abuse during pregnancy". Acta Paediatrica Scandinavica. 275: 112–121. doi:10.1111/j.1651-2227.1979.tb06170.x. PMID 291283.
- Clarren, S.K. (2005). A thirty year journey from tragedy to hope. Foreword to Buxton, B. (2005). Damaged Angels: An Adoptive Mother Discovers the Tragic Toll of Alcohol in Pregnancy. New York: Carroll & Graf. ISBN 0-7867-1550-2.
External links
| Classification | |
|---|---|
| External resources |
| Wikimedia Commons has media related to Fetal alcohol syndrome. |