Amniocentesis
Amniocentesis (also referred to as an amniotic fluid test) is a medical procedure[1] used primarily in prenatal diagnosis of chromosomal abnormalities and fetal infections,[2] as well as for sex determination. In this procedure, a small amount of amniotic fluid, which contains fetal tissues, is sampled from the amniotic sac surrounding a developing fetus. The fetal DNA is then examined for genetic abnormalities. The most common reason to have an "amnio" performed is to determine whether a fetus has certain genetic disorders or a chromosomal abnormality, such as Down syndrome. Amniocentesis (or another procedure, called chorionic villus sampling (CVS)) can diagnose these problems in the womb.[3] These prenatal examinations can prove helpful to expectant guardians as they allow for evaluating the fetal health status and the possibility of treatment feasibility.[4] An amniocentesis is performed when a woman is between 14 and 16 weeks gestation. Women who choose to have this test are primarily those at increased risk for genetic and chromosomal problems, in part because the test is invasive and carries a small risk of miscarriage. This process can be used for prenatal sex discernment and hence this procedure has legal restrictions in some countries.
| Amniocentesis | |
|---|---|
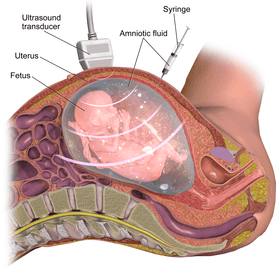 Amniocentesis | |
| Other names | Amniotic fluid test (AFT) |
| ICD-9-CM | 75.1 |
| MeSH | D000649 |
| MedlinePlus | 003921 |
History
Several researchers worked on the development of amniocentesis for fetal sex determination in the 1950s.[5]
Between 1959 and 1967,a scientist developed the new technique of amniocentesis for clinical assessment of fetal wellbeing in utero. He presented his results at the William Blair-Bell Memorial Lecture at the RCOG in London in 1965 and was awarded an MD from the University of Manchester for this work.[6] He also described amniocentesis techniques, as well as other details about amniotic fluid, in the chapter "The Liquor Amnii" in the 1970 and 1977 editions of Scientific Foundations of Obstetrics and Gynaecology.[7]
Up to the mid-1970s amniocentesis procedures were done 'blind‘. Doctors Jens Bang and Allen Northeved from Denmark were the first to report amniocentesis done with the guide of an ultrasound in 1972. Chorionic villus sampling (CVS) was first performed by Italian biologist Giuseppe Simoni in 1983. Real-time ultrasound is now used during all invasive procedures because it provides for the safety of the fetus and accuracy of results.
Medical uses
Genetic diagnosis
Early in pregnancy, amniocentesis is used for diagnosis of chromosomal and other fetal problems such as:[8]
- Down syndrome, also known as Trisomy 21
- Trisomy 13
- Trisomy 18
- Sex chromosome aneuploidies, also known as sex chromosome anomalies
- Fragile X
- Neural tube defects (anencephaly and spina bifida) by alpha-fetoprotein levels.[9]
- Rare, inherited metabolic disorders
Lung maturity
Amniocentesis can predict fetal lung maturity, which is inversely correlated to the risk of infant respiratory distress syndrome. Fetal lung maturity can be tested by sampling the amount of surfactant in the amniotic fluid in pregnancies greater than 30 weeks. Several tests are available, including the lecithin-sphingomyelin ratio ("L/S ratio"), the presence of phosphatidylglycerol (PG), or the surfactant/albumin (S/A) ratio.
- For the L/S ratio, if the result is less than 2:1, the fetal lungs may be surfactant deficient.
- The presence of PG usually indicates fetal lung maturity.
- For the S/A ratio, the result is given as mg of surfactant per gm of protein. An S/A ratio <35 indicates immature lungs, 35-55 is indeterminate, and >55 indicates mature surfactant production.
Infection
Amniocentesis can detect infections via decreased glucose level, a Gram stain showing bacteria, or abnormal differential count of white blood cells.[10]
Rh Incompatibility
Amniocentesis can be used to diagnose Rh incompatibility, a condition when the mother has Rh-negative blood and the fetus has Rh-positive blood. Early detection is important to treat the mother with Rh immune globulin and to treat her baby for hemolytic anemia.[11]
Decompression of polyhydramnios
Polyhydramnios, or the accumulation of amniotic fluids which leads to increase risk of cesarean section, can be relieved via decompression amniocentesis. Amniocentesis can also be used to diagnose potential causes of polyhydramnios.[12]
Preterm rupture of membranes
An emerging indication for amniocentesis is in the management of preterm rupture of membranes where measurement of certain amniotic fluid inflammatory markers may be helpful. If amniotic fluid IL-6, a marker of inflammation, is elevated, the fetus is at high risk and delivery should be considered.[13]
Risks
Amniocentesis is performed between the 15th and 20th week of pregnancy; performing this test earlier may result in fetal injury.[14] The term "early amniocentesis" is sometimes used to describe use of the process between weeks 11 and 13.[15]
Complications of amniocentesis include preterm labor and delivery, respiratory distress, postural deformities, chorioamnionitis, fetal trauma and alloimmunisation of the mother (rhesus disease). Studies from the 1970s originally estimated the risk of amniocentesis-related miscarriage at around 1 in 200 (0.5%).[16] Three more recent studies from 2000-2006 estimated the procedure-related pregnancy loss at 0.6-0.86%.[17] A more recent study (2006) has indicated this may actually be much lower, perhaps as low as 1 in 1,600 (0.06%).[18] Unlike the previous studies, the number in this study only reflects the loss that resulted from amniocentesis complications and excluded the cases when parents decided for an abortion following the test results.[17] In contrast to amniocentesis, the risk of miscarriage from chorionic villus sampling (CVS) is believed to be approximately 1 in 100, although CVS may be done up to four weeks earlier, and may be preferable if the possibility of genetic defects is thought to be higher.[19]
Amniotic fluid embolism has also been described as a possible.[20] Additional risks include amniotic fluid leakage and bleeding. These two are of particular importance because they can lead to spontaneous abortion in pregnant patients.[21]
Social implications
The prenatal diagnosis of chromosomal abnormalities can have social drawbacks as technology changes the way people think about disability and kinship. There is potential for intensification of attitudes of discrimination towards those with a disability, whose births could have been prevented through technology such as amniocentesis. In one sense, amniocentesis offers a window of control and in another, an anxiety-provoking responsibility to make rational decisions about complex, emotional and culturally contingent issues.[22][23]
Procedure
This procedure is typically performed in the outpatient setting by a team of providers. With ultrasound guidance, a needle is inserted into the abdomen at an angle through the muscle, into the uterus and into the amniotic cavity. There are various methods to obtain samples including a single needle and double needle technique. These techniques have their own variations in how they are performed including guidance of needle insertion location, and angle of needle insertion.[24] The collected amniotic fluid is then submitted for laboratory testing for chromosomal abnormalities and the puncture site heals with time.[24]
Ultrasound evaluation
Before the procedure is performed, the fetus is analyzed via ultrasound to determine if it is viable and for any abnormalities. The ultrasound determines the location of the placenta, fetal position and movements, and characteristics of the amniotic fluid. This information is utilized to determine the type of needle used and how the procedure should be performed.[25]
Preparation
The abdomen is treated and cleaned with antiseptic before the procedure. Sterile gel is also used on the abdomen before scanning with a sterile ultrasound probe. These measures are taken to reduce infection risk. The tools used in the procedure are coated with heparin to prevent clotting.[25]
Needle insertion
With the aid of ultrasound guidance, a needle is inserted through the mother's abdominal wall, then through the wall of the uterus, and finally into the amniotic sac. The physician then punctures the sac in an area away from the fetus and extracts approximately 20ml of amniotic fluid.[25] This procedure can be performed with a single needle or a double needle technique based on individualized patient factors and physician preference.[24] From the 20 ml of amniotic fluid, the first 2 ml is typically discarded due to mixture with maternal blood cells to ensure high quality fluid sampling.
Post procedure recommendations and analysis
If used for prenatal genetic diagnosis, fetal cells are separated from the extracted sample. The cells are grown in a culture medium, then fixed and stained. Under a microscope the chromosomes are examined for abnormalities. The most common abnormalities detected are Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Turner syndrome (monosomy X). The sample is also analyzed for fetal infection and for intra-amniotic inflammation through infection studies.[25]
After the procedure, the patient is instructed to take house rest for the initial 24 hours post-procedure however normal activities are allowed such as personal hygiene. In regard to the fetus, the puncture seals and the amniotic sac replenishes the liquid over the next 24–48 hours. One week after the procedure, the mother will have a follow up appointment for ultrasound analysis to confirm fetal viability and to assess healing of the puncture site.[25]
Stem cells
Amniotic fluid can be a rich source of multipotent mesenchymal, hematopoietic, neural, epithelial, and endothelial stem cells.[26][27][28]
A potential benefit of using amniotic stem cells over those obtained from embryos is that they side-step ethical concerns among pro-life activists by obtaining pluripotent lines of undifferentiated cells without harm to a fetus or destruction of an embryo. These stem cells would also, if used to treat the same individual they came from, sidestep the donor/recipient issue which has so far stymied all attempts to use donor-derived stem cells in therapies.
Artificial heart valves, working tracheas, as well as muscle, fat, bone, heart, neural and liver cells have all been engineered through use of amniotic stem cells.[29] Tissues obtained from amniotic cell lines show promise for patients suffering from congenital diseases/malformations of the heart, liver, lungs, kidneys, and cerebral tissue.[30]
The first amniotic stem cells bank in the US is active in Boston, Massachusetts.[31][32][33][34]
See also
- Chorionic villus sampling
- Amniotic fluid
- Amniotic stem cells
- Elective genetic and genomic testing
- Percutaneous umbilical cord blood sampling
- Prenatal diagnosis
References
- The word amniocentesis itself indicates precisely the procedure in question, Greek ἀμνίον amníon being the "inner membrane round the foetus" and κέντησις kéntēsis meaning "pricking", i.e. its puncture in order to retrieve some amniotic fluid.
- "Diagnostic Tests – Amniocentesis". Harvard Medical School. Archived from the original on 2008-05-16. Retrieved 2008-07-15.
- Carlson LM, Vora NL (June 2017). "Prenatal Diagnosis: Screening and Diagnostic Tools". Obstetrics and Gynecology Clinics of North America. 44 (2): 245–256. doi:10.1016/j.ogc.2017.02.004. PMC 5548328. PMID 28499534.
- Cheng WL, Hsiao CH, Tseng HW, Lee TP (August 2015). "Noninvasive prenatal diagnosis". Taiwanese Journal of Obstetrics & Gynecology. 54 (4): 343–9. doi:10.1016/j.tjog.2015.05.002. PMID 26384048.
- Barr ML (June 1956). "Prenatal Sex Determination". Canadian Medical Association Journal. 74 (11): 922–3. PMC 1824684. PMID 20325291.
- Robertson, I. (September 1992). "Campylobacter surveillance". Communicable Disease Report. CDR Weekly. 2 (36): 163. doi:10.1136/bmj.331.7526.1207. PMC 1285113. PMID 1285113.
- Philipp EE, Barnes J, Newton M, eds. (1970). Scientific Foundations of Obstetrics and Gynaecology. William Heinemann Medical Books (published 1977). pp. 285–291. ISBN 0-8151-6669-9.
- Prefumo F, Jauniaux E (January 2016). "Amniocentesis for fetal karyotyping: the end of an era?". BJOG. 123 (1): 99. doi:10.1111/1471-0528.13497. PMID 26715343.
- Dungan JS, Elias S (November 2008). "Prenatal Diagnostic Testing". The Merck Manuals Online Medical Library. Archived from the original on 4 August 2010. Retrieved July 30, 2010.
- Medina TM, Hill DA (February 2006). "Preterm premature rupture of membranes: diagnosis and management". American Family Physician. 73 (4): 659–64. PMID 16506709.
- "Rh Incompatibility | National Heart, Lung, and Blood Institute (NHLBI)". www.nhlbi.nih.gov.
- Zeino S, Carbillon L, Pharisien I, Tigaizin A, Benchimol M, Murtada R, Boujenah J (April 2017). "Delivery outcomes of term pregnancy complicated by idiopathic polyhydramnios". Journal of Gynecology Obstetrics and Human Reproduction. 46 (4): 349–354. doi:10.1016/j.jogoh.2017.02.014. PMID 28643663.
- Kenyon AP, Abi-Nader KN, Pandya PP (2010). "Pre-Term Pre-Labour Rupture of Membranes and the Role of Amniocentesis". Fetal and Maternal Medicine Review. 21 (2): 75–88. doi:10.1017/S096553951000001X.
- Seeds JW (August 2004). "Diagnostic mid trimester amniocentesis: how safe?". American Journal of Obstetrics and Gynecology. 191 (2): 607–15. doi:10.1016/j.ajog.2004.05.078. PMID 15343248.
- Sundberg K, Bang J, Smidt-Jensen S, Brocks V, Lundsteen C, Parner J, et al. (September 1997). "Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling". Lancet. 350 (9079): 697–703. doi:10.1016/S0140-6736(97)02449-5. PMID 9291904.
- Amniocentesis Risk Overrated?. Webmd.com (2006-11-01). Retrieved on 2011-11-22.
- Wilson RD, Langlois S, Johnson JA (July 2007). "Mid-trimester amniocentesis fetal loss rate". Journal of Obstetrics and Gynaecology Canada. 29 (7): 586–590. doi:10.1016/S1701-2163(16)32501-4. PMID 17623573.
- Eddleman KA, Malone FD, Sullivan L, Dukes K, Berkowitz RL, Kharbutli Y, et al. (November 2006). "Pregnancy loss rates after midtrimester amniocentesis". Obstetrics and Gynecology. 108 (5): 1067–72. doi:10.1097/01.AOG.0000240135.13594.07. PMID 17077226.
- Rhoads GG, Jackson LG, Schlesselman SE, de la Cruz FF, Desnick RJ, Golbus MS, et al. (March 1989). "The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities". The New England Journal of Medicine. 320 (10): 609–17. doi:10.1056/NEJM198903093201001. PMID 2645520.
- Dodgson J, Martin J, Boswell J, Goodall HB, Smith R (May 1987). "Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion". British Medical Journal. 294 (6583): 1322–3. doi:10.1136/bmj.294.6583.1322. PMC 1246486. PMID 3109636.
- Tara F, Lotfalizadeh M, Moeindarbari S (August 2016). "The effect of diagnostic amniocentesis and its complications on early spontaneous abortion". Electronic Physician. 8 (8): 2787–2792. doi:10.19082/2787. PMC 5053461. PMID 27757190.
- Lock M, Nguyen V (2010). An Anthropology of Biomedicine. Oxford: Wiley-Blackwell.
- Rapp R (1998). "Refusing prenatal diagnosis: the meanings of bioscience in a multicultural world". Science, Technology & Human Values. 23 (1): 45–70. doi:10.1177/016224399802300103. PMID 11660551.
- Monni G, Pagani G, Stagnati V, Iuculano A, Ibba RM (2016-05-02). "How to perform transabdominal chorionic villus sampling: a practical guideline". The Journal of Maternal-Fetal & Neonatal Medicine. 29 (9): 1499–505. doi:10.3109/14767058.2015.1051959. PMID 26372474.
- Cruz-Lemini M, Parra-Saavedra M, Borobio V, Bennasar M, Goncé A, Martínez JM, Borrell A (December 2014). "How to perform an amniocentesis". Ultrasound in Obstetrics & Gynecology. 44 (6): 727–31. doi:10.1002/uog.14680. PMID 25449117.
- Weiss, Rick (2007-01-08). "Scientists See Potential In Amniotic Stem Cells". The Washington Post. Retrieved 2010-04-23.
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, et al. (January 2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biotechnology. 25 (1): 100–6. doi:10.1038/nbt1274. PMID 17206138.
- "Stem Cells – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
- Bajek A, Olkowska J, Gurtowska N, Kloskowski T, Walentowicz-Sadlecka M, Sadlecki P, et al. (June 2014). "Human amniotic-fluid-derived stem cells: a unique source for regenerative medicine". Expert Opinion on Biological Therapy. 14 (6): 831–9. doi:10.1517/14712598.2014.898749. PMID 24655038.
- "Stem cells scientific updates – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
- "European Biotech Company Biocell Center Opens First united state Facility for Preservation of Amniotic Stem Cells in Medford, Massachusetts | Reuters". 2009-10-22. Archived from the original on October 30, 2009. Retrieved 2010-01-11.
- "Europe's Biocell Center opens Medford office – Daily Business Update – The Boston Globe". 2009-10-22. Archived from the original on 12 January 2010. Retrieved 2010-01-11.
- "The Ticker - BostonHerald.com". Retrieved 2010-01-11.
- "Biocell partner with largest New England's hospital group to preserve amniotic stem cell". Archived from the original on 14 March 2010. Retrieved 2010-03-10.
External links

- Amniocentesis (medical procedure) at the Encyclopædia Britannica
- Amniodex is an interactive decision support intervention designed for women faced with the decision of whether to undergo amniocentesis.
- The Amniocentesis Report A Decision Guide for Expectant Parents and Health Care Professionals