Guidance for US Laboratories Testing for Zika Virus Infection
July 24, 2017
On This Page
- Overview of Zika Virus Testing
- Overview of Updates to Testing Guidance
- Biological Safety
- Testing methods
- Detailed Information for Specimen Types for Zika Testing
- Reporting
- References
- Table 1. Results Interpretation for Pregnant Women
- Table 2. Results Interpretation for Non-Pregnant Individuals
- Figure 1. Testing Algorithm for Symptomatic Non-Pregnant Individuals
This page also available as a PDF
Overview of Zika Virus Testing
Current guidance for US laboratories testing for Zika virus infection recommends that testing be limited to specimens collected from patients meeting CDC’s clinical and epidemiologic criteria for testing1. Clinical signs and symptoms associated with Zika virus infection are discussed here. Current Zika information and guidance is available on CDC’s Zika website. Information specific to laboratories is available.
Current information and guidance specific to Zika virus in Puerto Rico can be found at the Puerto Rico Department of Health website.
Multiple assays and sample types are often needed to establish a definitive laboratory diagnosis of Zika virus infection due to the temporal nature of biologic analytes in the infected person. Viral ribonucleic acid (RNA) is the first analyte that can be detected in an infected person in multiple specimen types. As the immune response develops, immunoglobulin M (IgM) titers rise in peripheral blood and the level of viral RNA generally declines. However, viral RNA may be detectable in some infected people for longer periods in certain specimen types. Nucleic acid testing (NAT) is most informative in the first 6 weeks after symptom onset. IgM antibodies are most likely to be detected in the first 12 weeks after symptom onset, but may persist longer.
Zika virus infection can cause signs and symptoms similar to those seen in patients with other arthropod-borne virus (arbovirus) infections, including dengue viruses, related flaviviruses, and chikungunya virus, an unrelated alphavirus. For differential diagnosis of Zika virus infection, testing for other circulating arboviruses/flaviviruses should be considered. A positive result for one of these viruses does not preclude infection with the others. Co-infection with Zika virus and dengue or chikungunya viruses is rare, but possible, particularly in areas where these viruses are co-circulating.
Paired serum and urine are the primary diagnostic specimens for Zika virus infection. Other specimen types such as plasma, whole blood, cerebrospinal fluid (CSF), and amniotic fluid are authorized for use with some assays that have received an Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA). For all diagnostic testing conducted on specimen types other than patient-matched serum and urine, it is necessary to concurrently obtain a patient-matched serum specimen for NAT and/or IgM (serology) testing, as appropriate. Please review assay instructions to determine acceptable specimen types for a given assay. Instructions for use of assays authorized for diagnostic use under an FDA EUA can be found under the “Labeling” bullet for each assay on the FDA website.
This updated guidance makes the following recommendations for Zika virus testing in:
- Symptomatic pregnant women with possible exposure to Zika virus. See areas with risk of exposure.
- Asymptomatic pregnant women with ongoing possible exposure to Zika virus
- Pregnant women with possible exposure to Zika virus who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection
- Non-pregnant symptomatic individuals with possible exposure to areas with risk of Zika virus transmission
Zika virus testing may be considered for:
- Asymptomatic pregnant women with recent possible but no ongoing exposure to Zika virus (i.e., travelers). Although not routinely recommended, testing may be considered on a case-by case basis and in line with jurisdictional recommendations.
Zika virus testing is not recommended for:
- Non-pregnant asymptomatic individuals
- Pre-conception screening
Laboratories should complete ALL specimen testing, including any indicated repeat testing, before reporting test results to provider. Clinical decisions surrounding patient management should not be made until the appropriate testing algorithm is completed.
Overview of Updates to Testing Guidance
The Updated Interim Guidance for Healthcare Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2017 can be found here. The interim guidance has been updated based on declining trends in the number of reported cases of Zika virus infection in Region of the Americas, emerging evidence on prolonged detection of Zika immunoglobulin M (IgM) antibodies, and new limitations for interpreting serologic tests during pregnancy. Although IgM is most likely to be detected in the first 12 weeks after infection, emerging data indicate that Zika virus IgM may persist beyond 12 weeks in a subset of infected individuals, limiting the ability of testing to determine whether an infection occurred during or prior to pregnancy. IgM tests are also susceptible to false positives and cross-reactivity with other flaviviruses, especially when an individual has been vaccinated against or previously infected with a related flavivirus. In the United States, as the decline in reported Zika virus cases (including travel-associated cases) continues, the proportion of positive tests that are false positives for Zika virus are expected to increase due to a low positive predictive value. Key changes in the updated guidance for testing pregnant women have been made taking these testing limitations into consideration.
Key updates to the guidance:
- Symptomatic pregnant women with possible Zika virus exposure should be tested. When testing symptomatic pregnant women, concurrent NAT and IgM testing is recommended as soon as possible, up to 12 weeks after symptom onset. The recommendation for Zika virus NAT testing has been expanded from ≤ 2 weeks to ≤ 12 weeks because of evidence that Zika virus RNA may persist in the serum of pregnant women with Zika virus infection. NAT testing is now recommended on paired serum AND urine specimens collected at the same visit.
- Asymptomatic pregnant women with ongoing possible exposure to Zika virus should be tested. NAT testing is recommended three times during pregnancy. IgM serology testing is not routinely recommended. Recommendations for the timing of NAT testing are at the initial prenatal care visit, followed by two additional NAT tests performed during pregnancy, coinciding with non-consecutive prenatal visits. Timing of additional NAT testing may be informed by jurisdictional trends in Zika virus transmission, the expected length of Zika virus nucleic acid detection in serum, and the duration of exposure during pregnancy. Although not routinely recommended, after pre-test counseling and individualized risk assessment, physicians and patients, through a shared decision-making model, may collaboratively elect to have IgM testing performed concurrent with NAT testing. For women who have a positive NAT test during pregnancy, additional NAT testing is not recommended. If a patient has previously been confirmed positive for Zika virus infection, no additional IgM serology testing is recommended.
- Asymptomatic pregnant women with recent possible Zika virus exposure but no ongoing exposure (i.e., travelers) may be considered for testing. Although not routinely recommended, testing may be considered on a case-by-case basis using a shared physician-patient decision-making model and in line with jurisdictional recommendations. If testing of asymptomatic pregnant women is performed, the same algorithm as for symptomatic pregnant women should be followed using the timeframe from the last possible exposure to Zika virus.
- Pregnant women with possible exposure to Zika virus and who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection should be tested. NAT and IgM testing should be performed on maternal serum and urine following the algorithm for symptomatic pregnant women. If amniocentesis is being performed as part of clinical care, NAT testing of amniocentesis specimens should also be performed. Testing of placental and fetal tissues may also be considered.
Testing guidance for symptomatic non-pregnant individuals remains unchanged with this updated interim guidance. Pre-conception screening and baseline serum screening are not recommended. There are currently no EUA-approved tests for screening of semen for male partners requesting screening.
Information regarding testing infants at the time of birth can be found on CDC’s Zika website and also in the Interim Guidance for the Evaluation and Management of Infants with Possible Congenital Zika Virus Infection, or here.
Biological Safety
To ensure laboratory safety when working with Zika virus, please review CDC guidance on Transport and Handling of Diagnostic Specimens and Working with Zika Virus in the Laboratory. See Biosafety in Microbiological and Biomedical Laboratories (BMBL) for additional biosafety information about arboviruses and laboratory biosafety practices.
Testing Methods
- Additional volume of serum beyond what is recommended in EUAs should be obtained in case repeated NAT testing is indicated by initial test results.
- The updated interim guidance recommends that NAT and IgM serology testing be performed concurrently when testing symptomatic pregnant women.
- The updated interim guidance recommends that NAT testing be performed concurrently on both serum AND a paired urine specimen when testing symptomatic pregnant women.
- When the risk of exposure to Zika virus is low, the incidence of false positive test results is increased for both NAT and IgM serology testing. Further, IgM testing does not provide a clear determination of the timing of exposure, and a positive IgM result may represent recent or persistent IgM response to Zika virus or another flavivirus infection.
- Given the possibility of a false positive result, laboratory test results should not be released until all testing is complete.
- It is recommended that healthcare decisions not be made until testing according to the appropriate algorithm is complete.
Molecular Testing
Nucleic acid test, or NAT, is a generic term referring to all molecular tests used to detect viral genomic material. Despite the specificity of molecular testing, false positive NAT results have been reported in rare cases and may depend on the type of NAT assay performed and patient population (i.e., limited or no prevalence of viral transmission) being tested. This problem can be exacerbated when Zika virus testing is performed on patient populations not recommended in the Zika testing algorithms. Testing is not recommended for asymptomatic non-pregnant individuals, or for pre-conception screening for either the woman or her partner.
Under updated recommendations, repeat NAT testing of the same specimen beginning with a new extraction is recommended in some circumstances. To reduce the loss of detectable viral RNA in urine specimens, urine should only be stored at 4 ˚C for ≤ 48 hours, and any indicated repeat NAT testing of urine specimens should be performed within that time.
Updated recommendations for NAT testing of pregnant women are as follows:
Symptomatic Pregnant Women
For symptomatic pregnant women with possible exposure to Zika virus, NAT and IgM serology testing for Zika virus should be performed concurrently (i.e., in parallel) on specimens collected as soon as possible, up to 12 weeks post-symptom onset. Patients should also be evaluated for other pathogens (including dengue virus) circulating in areas where they have traveled or lived. Possible exposure may include travel to or residence in areas with increased risk of Zika virus infection or unprotected sex with a partner who has traveled to or lives in an area with increased risk of Zika virus exposure. See areas with risk of exposure.
IgM antibodies usually become detectable within a week following symptom onset and decline over time, therefore a negative IgM assay result on a serum specimen collected less than 2 weeks before or more than 12 weeks after symptom onset does not rule out a recent Zika virus infection. A positive NAT result with a concurrent negative IgM result on a specimen <14 days post symptom onset or most recent exposure may reflect specimen collection before the development of detectable antibodies or, in rare cases, a false positive assay result. Despite the specificity of NAT, false positive NAT results have been reported. If both serum and urine test positive for Zika virus, repeat NAT testing is not required (see Table 1). If NAT is only positive on serum or urine, and IgM antibody testing is negative, NAT should be repeated on the original, positive specimen beginning with a new extraction. If the quantity of specimen is not sufficient for repeat NAT testing or if the original NAT is positive and repeat NAT is negative, IgM testing is recommended on a serum specimen collected ≥ 2 weeks after date of most recent specimen draw. For women who are NAT positive during pregnancy, additional NAT testing is not recommended. If more than 12 weeks have passed since symptom onset, IgM serology testing without NAT may be considered but a negative IgM result does not rule out recent infection.
Asymptomatic Pregnant Women
Testing is recommended for asymptomatic pregnant women with ongoing possible exposure to Zika virus. Ongoing exposure may include daily travel to or residence in areas with risk of Zika virus infection or unprotected sex with a partner who travels to or resides in an area with risk of Zika virus exposure. See areas with risk of exposure. NAT testing for evidence of Zika virus infection is recommended three times during pregnancy, but IgM serology testing is not routinely recommended. Recommendations for the timing of NAT testing are at the initial prenatal care visit, followed by two additional NAT tests, the timing and frequency of which are at the discretion of the provider and dependent on the risk of exposure and the results of the initial NAT test. If the initial NAT test is positive, no additional NAT tests are recommended during pregnancy. While not routinely recommended, after pre-test counseling, physicians and patients may elect to have IgM testing performed concurrently with NAT testing. For women who have a positive NAT result during pregnancy, additional NAT testing is not recommended. If a patient has previously been confirmed positive for Zika virus infection, no additional IgM serology testing is recommended. If both serum and urine test positive for Zika virus, repeat NAT testing is not required (see Table 1).
Testing of asymptomatic pregnant women with recent possible Zika virus exposure but no ongoing exposure (i.e., travelers) should be considered on a case–by-case basis and in line with jurisdictional recommendations. If testing is conducted, the testing algorithm for symptomatic pregnant women should be followed, with specimens collected as soon as possible, not to exceed 12 weeks from the last possible exposure.
Pregnant Women with Prenatal Ultrasound Findings Consistent with Congenital Zika Virus Infection
Pregnant women with possible exposure to Zika virus who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection should be tested. Possible exposure may include travel to or residence in areas with risk of Zika virus infection or sex with a partner who has traveled to or lives in an area with risk of Zika virus exposure. See areas with risk of exposure. NAT and IgM testing should be performed on maternal serum and urine following the algorithm for symptomatic pregnant women. If amniocentesis is being performed as part of clinical care, NAT testing of amniocentesis specimens should also be performed. Data regarding the utility of amniotic fluid in diagnosing congenital Zika virus infection are limited.
Symptomatic Non-Pregnant Individuals
NAT testing of symptomatic non-pregnant individuals is dependent on the timing of specimen collection. Zika virus and dengue virus NAT testing should be performed on specimens collected < 14 days after symptom onset (Figure 1). Zika virus and dengue virus IgM serology testing should be performed on NAT negative samples collected <14 days after onset of symptoms or on samples collected ≥ 14 days after onset of symptoms, and NAT testing is not recommended on specimens collected ≥ 14 days after symptom onset. NAT testing is not recommended for asymptomatic non-pregnant individuals.
Additional Molecular Test Information
Multiple NATs have received EUA from FDA. Most, but not all, of the NATs that have received EUAs from FDA are rRT-PCR-based. FDA maintains a list on its website of all Zika virus EUAs. Please refer to the FDA website for a current list of authorized assays and associated letters of authorization, fact sheets, and product labeling. Additional assay-specific information (e.g., performance characteristics) is included in the labeling for the assay.
Information about molecular assays that have been cleared by FDA for detection of arboviruses other than Zika virus can be found in this searchable database.
Antibody Detection Methods
Due to the temporal nature of Zika virus RNA in serum and urine, a negative NAT does not exclude recent Zika infection. Serologic detection of Zika virus infection may help confirm exposure to Zika virus in settings where people have not previously been exposed to Zika virus. Antibodies (IgM) directed against Zika virus are typically first detected as viral RNA begins to wane. The decline in reported cases of Zika virus infection in the Americas in 2017 compared to 2016 is expected to increase the proportion of false positive test results for Zika virus. Although IgM is most likely to be detected in the first 12 weeks after infection, emerging data indicate that Zika virus IgM may persist beyond 12 weeks in a subset of infected individuals, limiting the ability of testing to determine whether an infection occurred during or prior to pregnancy. These limitations are a particular challenge when Zika virus testing is performed on patient populations not recommended in the Zika testing algorithms. Testing is not recommended for asymptomatic non-pregnant individuals or for pre-conception screening. The updated testing algorithms recommend serologic testing for the following patient populations:
Pregnant Women
For symptomatic pregnant women, NAT and IgM serology for Zika virus and dengue virus should be performed concurrently (i.e., in parallel) on specimens collected ≤ 12 weeks after symptom onset. IgM antibodies decline over time. Therefore, a negative IgM assay result does not rule out a recent Zika virus infection.
For asymptomatic pregnant women, if testing is conducted, the same testing recommendation should be followed as for symptomatic pregnant women, on specimens collected ≤ 12 weeks from the last possible exposure to Zika virus. Testing for other arboviral pathogens is not routinely recommended for asymptomatic pregnant women.
Non-Pregnant Symptomatic Individuals
For non-pregnant symptomatic individuals, specimens collected from > 14 days to < 12 weeks after symptom onset should be tested using anti-Zika IgM and anti-dengue IgM as the recommended initial assay. IgM testing is not recommended for non-pregnant asymptomatic individuals (Figure 1).
Additional Serologic Test Information
For explanation of a specific interpretation on Zika virus IgM serology assays, refer to the instructions for use for the specific assay performed. Information on each assay can be found on FDA’s website, under the “Labeling” bullet for the specific assay. The Updated Interim Guidance for Healthcare Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2017, contains specific information that guides the overall interpretation of combined results from Zika virus and dengue virus serology and PRNT. This information may also be found in Tables 1 and 2.
Zika virus IgM assays give a presumptive positive result, and final assay interpretation depends on additional results from confirmatory testing, such as the plaque reduction neutralization test (PRNT). Given the possibility of a false positive result, most likely due to cross reactivity, which has been reported, it is recommended that healthcare decisions are not made until the testing algorithm is complete. PRNT testing is recommended for serum specimens that yield a non-negative IgM serology result. However, PRNT may also reflect prior infection, and cannot be used to determine the timing of Zika virus infection. Terminology indicating a non-negative IgM serology result varies by assay, and may include positive, equivocal, presumptive, or possible Zika virus results.
Neutralizing antibodies develop shortly after IgM antibodies and likely persist for many years (22). Based on experience with other flaviviruses, previous Zika virus infection is likely to confer prolonged, possibly lifelong, immunity (23). Testing is not routinely recommended for pregnant women who have been previously diagnosed with confirmed Zika virus infection by either NAT or serology (Zika IgM positive/equivocal and Zika PRNT ≥ 10 and Dengue PRNT <10). However, for pregnant women without a previous definitive diagnosis of Zika virus infection (e.g., pregnant women with laboratory evidence of recent flavivirus infection or laboratory evidence of presumptive Zika or flavivirus infection), given the limitations of serology testing (e.g., cross-reactivity and false positive test results), decisions about testing during a subsequent pregnancy should be made using a shared patient-provider decision-making model. If the decision is made to test, only NAT testing is recommended, because IgM antibody test might not be able to determine the timing of infection among pregnant women who had exposure to Zika virus before the current pregnancy.
Zika and dengue viruses have similar clinical presentations, transmission cycles, and geographic distributions, and cross-reactivity on serologic assays for these viruses is common. Dengue IgM testing should be performed on any symptomatic person with possible dengue exposure so they can receive appropriate clinical management; dengue virus IgM testing is not recommended for asymptomatic pregnant women or asymptomatic non-pregnant individuals. Currently, for one FDA–authorized Zika IgM assay, follow-up testing is to be done with an FDA-cleared dengue IgM assay when the final interpretation is “Presumptive Other Flavivirus Positive” due to the inclusion of a cross-reactive control that includes a dengue virus antigen. For this same assay, follow-up testing is to be done with an FDA-cleared West Nile virus IgM assay when the final interpretation is “Presumptive Other Flavivirus Positive,” as the other component of the cross-reactive control is West Nile virus antigen. For people who were in regions with known endemic flavivirus activity (e.g., West Nile virus, St. Louis encephalitis virus) during their potential exposure period, IgM testing for those viral infections should be considered using an FDA-cleared assay, if available. See more information about West Nile virus. Because infections with other arboviruses, including chikungunya virus, can also produce symptoms similar to Zika virus infection, additional testing for other arboviruses is often needed to reach a diagnosis. For people with chikungunya virus exposure risk and a clinically compatible illness2, anti-chikungunya IgM testing should also be performed.
FDA maintains on its website a list of all Zika virus EUAs. Please refer to the FDA website for a current list of authorized assays and associated letters of authorization, fact sheets, and product labeling. Assay-specific information (e.g., performance characteristics) is included in the labeling for each assay.
Confirmation of Anti-Zika IgM Reactive Results and Anti-Dengue IgM Reactive Results by Plaque Reduction Neutralization Test (PRNT)
Zika virus IgM assays yield a presumptive positive result, and final assay interpretation depends on additional results from confirmatory testing, such as the PRNT. PRNT measures virus-specific neutralizing antibodies to Zika virus and other endemic flaviviruses. Currently, within the United States and most US territories, when IgM serology indicates the potential presence of anti-Zika IgM antibodies, PRNT is needed to confirm diagnosis. If ELISA testing indicates a positive or equivocal result for dengue virus infection, confirmatory testing is to be performed as indicated in the IgM assay labeling. PRNT is performed at CDC, a laboratory designated by the CDC (i.e., a laboratory that has independently demonstrated proficiency to perform PRNT testing by completing a proficiency panel provided by the CDC), or a public health laboratory (PHL) PRNT reference center. Confirmatory testing can take up to 4 weeks, and PRNT results will be reported via the laboratory to which the original specimen was submitted.
Given the high degree of antibody cross-reactivity observed with Zika virus and dengue virus infections, results of Zika/dengue PRNT testing should be interpreted alongside the initial IgM assay results to assess the timing of infection. PRNT is not always able to provide a definitive determination of the specific flavivirus causing a recent infection, particularly in people with a prior history of flavivirus infection. For this reason, PRNT confirmation is not routinely recommended for people living in areas with high levels of circulating flaviviruses (e.g., dengue).
The Updated Interim Guidance for Healthcare Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2017 and Tables 1 and 2 below contain specific information that guides the overall interpretation of combined results from Zika virus and dengue virus serology and PRNT.
For additional information, please refer to CDC guidance on clinical management of patients with positive assay results.
Detailed Information for Specimen Types for Zika Testing
Detailed characteristics of the various specimen types that have been validated for use with Zika virus diagnostic assays can be found on FDA’s website and CDC’s Zika website. Individual FDA-authorized assays have specific information concerning specimen handling and storage. It is important to note that all diagnostic algorithms for pregnant women are for patient-matched serum and urine, and a patient-matched serum specimen must be submitted alongside all other sample types for follow-up testing, if needed. Consider collecting additional volume beyond what is suggested in EUAs, to permit for potential repeat specimen testing.
Tissue Specimens
There are currently no FDA-authorized assays for Zika virus testing of tissue specimens, including fetal and placental tissue. Requests for testing should be coordinated through state or local health departments and pre-approval is required before submission to CDC. See additional information about specimen collection and submission procedures.
Other Specimens Types
There are currently no FDA-authorized Zika virus assays for which performance with other specimen types, such as semen and saliva, has been established.
Specimen Referral
Healthcare and laboratory professionals should direct Zika virus testing requests to their local or state public health laboratory or to a commercial laboratory that performs Zika virus testing using an FDA-authorized assay. Healthcare and laboratory professionals should follow state or local public health department guidance on notification procedures for suspect cases of Zika virus infection.
Public health laboratories that do not perform Zika virus testing should work with their state, local, or territorial public health department for testing of suspect specimens or referring specimens to CDC.
When submitting specimens for Zika virus testing, indicate pregnancy status and exposure risk of patient to ensure appropriate testing algorithm is followed.
For questions about testing within Puerto Rico, please call 787-706-2399. For submission of specimens, please submit a dengue case investigation report (DCIR) for each specimen, which can be downloaded here.
Reporting
Clinical decisions surrounding patient management should not be made until the appropriate testing, according to the most recent algorithm, is completed. Recommended testing may include repeated NAT testing, serology, and PRNT testing, thus providers may receive multiple NAT and serology test reports from more than one testing laboratory before all testing of a patient specimen is completed. Further, jurisdictional testing may vary from CDC recommended guidelines. For guidance on final result interpretation, healthcare providers should consult with their local jurisdiction.
Laboratories should complete specimen testing, including any indicated repeat testing, before submitting test results to providers. Assay results generated for each specimen should be reported to clinicians as specified in the assay instructions for use. Reports should include language indicating that clinical decisions surrounding patient management should not be made until all testing is complete and should be considered within the context of all test results, and clinical and epidemiologic criteria as specified for the testing algorithm appropriate for the person being tested.
Laboratories must include the appropriate fact sheets with each FDA-authorized test result when reporting back to providers and patients. Fact sheets have been prepared for healthcare providers and patients to help them understand the results of testing. Authorized fact sheets for each assay under an EUA are posted to the FDA website.
In addition, laboratories must collect information on the performance of any of the EUA Zika assays and report to FDA (via email: CDRH-EUA-Reporting@fda.hhs.gov) and the assay manufacturer any occurrence of false positive or false negative results of which they become aware.
Please note that Zika, dengue, West Nile, and chikungunya virus infections are all on the 2017 list of nationally notifiable conditions. Therefore, test results should be reported back to state or local health department staff to facilitate investigation and classification of the case and reporting to CDC.
Guidance documents are available to assist in applying laboratory results to determine patient care and patient follow-up decisions:
- Zika clinical guidance for healthcare providers caring for pregnant women, women of reproductive age, infants, children, or other symptomatic individuals
- Dengue clinical guidance
- Chikungunya clinical guidance
- West Nile clinical guidance
References
Oduyebo, T, Polen, K, Walke, H, et al. Updated Interim Guidance for Healthcare Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2017.
1The term “clinical and/or epidemiologic criteria” refers to factors such as symptoms, pregnancy, and exposure risk. Please refer to current CDC clinical guidance.
2Clinical information about chikungunya virus infection, including clinical evaluation guidance, may be found on CDC’s website.
Table 1. Interpretation of results of nucleic acid and antibody testing for suspected Zika virus infection*,†, §, ¶— United States, 2017
| Zika NAT (serum)** | Zika NAT (urine)** | Zika virus and dengue virus IgM†† | Zika virus PRNT | Dengue virus PRNT | Interpretation and recommendations |
|---|---|---|---|---|---|
| Positive | Positive | Any result | Not indicated | Not indicated | Acute Zika virus infection |
| Negative | Positive | Positive | Not indicated | Not indicated | Acute Zika virus infection |
| Negative | Positive | Negative | Not indicated | Not indicated | Suggests acute Zika virus infection Repeat testing on original urine specimen. If repeat NAT result is positive, interpret as evidence of acute Zika virus infection If repeat NAT result is negative, repeat Zika virus IgM antibody testing on a serum specimen collected ≥2 weeks after onset or possible exposure or specimen collection date. If repeat IgM antibody result is positive§§, interpret as evidence of acute Zika virus infection. If repeat IgM result is not positive, interpret as no evidence of Zika virus infection. |
| Positive | Negative or not performed | Positive | Not indicated | Not indicated | Acute Zika virus infection |
| Positive | Negative or not performed | Negative | Not indicated | Not indicated | Suggests Acute Zika virus infection Repeat testing on original serum specimen. If repeat NAT result is positive, interpret as evidence of acute Zika virus infection. If repeat NAT result is negative, repeat Zika virus IgM antibody testing on a serum specimen collected ≥2 weeks after onset or possible exposure or specimen collection date. If repeat IgM antibody result is positive§§, interpret as evidence of acute Zika virus infection. If repeat IgM antibody result is not positive, interpret as no evidence of Zika virus infection. |
| Negative | Negative or not performed | Any non-negative result¶¶ | ≥10 | <10 | Zika virus infection, timing of infection cannot be determined. For persons without prior Zika virus exposure, a positive IgM result represents recent Zika virus infection. |
| Negative | Negative or not performed | Any non-negative result¶¶ | <10 | Any result | No evidence of Zika virus infection. |
| Negative | Negative or not performed | Any non-negative result¶¶ | ≥10 | ≥10 | Flavivirus infection; specific virus cannot be identified, timing of infection cannot be determined. For persons without prior Zika virus exposure, a positive IgM result represents recent unspecified flavivirus infection. |
| For areas where PRNT is not recommended¶ | |||||
| Negative | Negative or not performed | Positive for Zika virus AND negative for dengue virus | Not performed because PRNT is not recommended | Presumptive Zika virus infection; timing of infection cannot be determined. *** | |
| Negative | Negative or not performed | Positive for Zika virus AND positive for dengue virus | Not performed because PRNT is not recommended | Presumptive flavivirus infection; specific virus cannot be identified; timing of infection cannot be determined. *** | |
| Negative | Negative or not performed | Equivocal (either or both assays) | Not performed because PRNT is not recommended | Insufficient information for interpretation. Consider repeat testing. | |
| Negative | Negative or not performed | Negative on both assays | Not performed because PRNT is not recommended | No laboratory evidence of Zika virus infection. | |
Abbreviations: IgM = immunoglobulin M antibodies; NAT= nucleic acid test; PRNT = plaque reduction neutralization test.
* Final interpretations of results of Zika virus tests should be performed after all testing is complete.
† Serology test results that indicate recent flavivirus infection should be interpreted in the context of the circulating flaviviruses.
§ Dengue virus IgM antibody testing is recommended for symptomatic pregnant women as well as for pregnant women residing in areas where PRNT confirmation is not recommended.
¶ Currently, PRNT confirmation is not routinely recommended for persons living in Puerto Rico.
** Serum must be submitted for all persons tested for Zika virus infection; urine specimen for Zika virus NAT testing should always be submitted concurrently with a serum specimen.
†† For laboratory interpretation in the presence of dengue virus IgM results, refer to CDC’s Laboratory Guidance for Dengue.
§§ Positive results include “positive,” “presumptive Zika virus positive,” or “possible Zika virus positive.” These are examples of assay interpretations that might accompany test results; positive serology terminology varies by assay. For explanation of a specific interpretation, refer to the instructions for use of the specific assay performed. See information on each assay on FDA’s website under “Labeling” for the specific assay.
¶¶ Nonnegative results include “positive,” “equivocal,” “presumptive positive,” or “possible positive.” These are examples of assay interpretations that might accompany test results; nonnegative serology terminology varies by assay. For explanation of a specific interpretation, refer to the instructions for use for the specific assay performed. See information on each assay on FDA’s website under “Labeling” for the specific assay.
*** Zika virus IgM positive result is reported as “presumptive positive or flavivirus infection” to denote the need to perform confirmatory PRNT titers against Zika virus, dengue virus, and other flaviviruses to which the person might have been exposed, to resolve potential false-positive results that might have been caused by cross-reactivity or nonspecific reactivity. In addition, ambiguous test results (e.g., inconclusive, equivocal, and indeterminate) that are not resolved by retesting also should have PRNT titers performed to rule out false-positive result. However, PRNT confirmation is currently not routinely recommended for persons living in Puerto Rico.
Table 2. Interpretation of results of nucleic acid and antibody testing for suspected Zika virus infection in non-pregnant individuals*,†, §, ¶, **,†† — United States, 2017
Specimen collected < 14 days post-symptom onset
| Zika NAT; urine | Zika NAT; serum | Zika virus IgM¶ | Zika virus PRNT | Dengue virus PRNT | Interpretation Lab results should not be released until testing is complete |
|---|---|---|---|---|---|
| Positive | Positive | Not indicated | Not indicated | Acute Zika virus infection. | |
| Positive | Negative | Not indicated | Not indicated | Acute Zika virus infection. | |
| Negative or not performed | Positive | Not indicated | Not indicated | Acute Zika virus infection. | |
| Negative or not performed | Negative | Negative | Not indicated | No laboratory evidence of Zika virus infection. | |
| Specimen collected < 14 days post-symptom onset with negative NAT, or specimen collected ≥ 14 days post-symptom onset | |||||
| Negative or not performed | Negative or not performed | Any non-negative result | ≥10 | <10 | Zika virus infection, timing of infection cannot be determined. A positive IgM and PRNT result for Zika and a negative PRNT for dengue likely represents recent Zika infection. |
| Negative or not performed | Negative or not performed | Any non-negative result | <10 | Any result | No evidence of Zika virus infection. |
| Negative or not performed | Negative or not performed | Any non-negative result | ≥10 | ≥10 | Flavivirus infection; specific virus cannot be identified, precise timing of infection cannot be determined. |
| Negative or not performed | Negative or not performed | Any non-negative result | Pending | Presumptive Zika virus infection; timing of infection cannot be determined. For patients with no exposure prior to testing, a positive IgM result represents recent Zika infection. However, without PRNT confirmatory testing, false positives cannot be ruled out. | |
Abbreviations: IgM = immunoglobulin M antibodies; NAT= nucleic acid test; PRNT = plaque reduction neutralization test.
* Serology test results that indicate recent flavivirus infection should be interpreted in the context of the currently circulating flaviviruses.
† Examples of assay interpretations that are positive include positive, or presumptive Zika virus positive, or possible Zika virus positive. For explanation of a specific interpretation, refer to the instructions for use for the specific assay performed. See information on each assay on FDA’s website under “Labeling” for the specific assay.
§ Serum must be submitted for all persons tested for Zika virus infection; urine specimen for Zika virus testing should always be submitted with a paired serum specimen.
¶Zika virus IgM positive result is reported as “presumptive positive” to denote the need to perform confirmatory PRNT.
**Currently, PRNT confirmation is not routinely recommended for individuals living in Puerto Rico.
††To resolve false-positive results that might be caused by cross-reactivity or nonspecific reactivity, presumptive positive Zika virus IgM results should be confirmed with PRNT titers against Zika virus, dengue virus, and other flaviviruses to which the person might have been exposed. In addition, ambiguous test results (e.g. inconclusive, equivocal, and indeterminate) that are not resolved by retesting also should have PRNT titers performed to rule out a false-positive result.
Figure 1. Testing Recommendations for Symptomatic Non-Pregnant Individuals with Exposure to Zika Virus
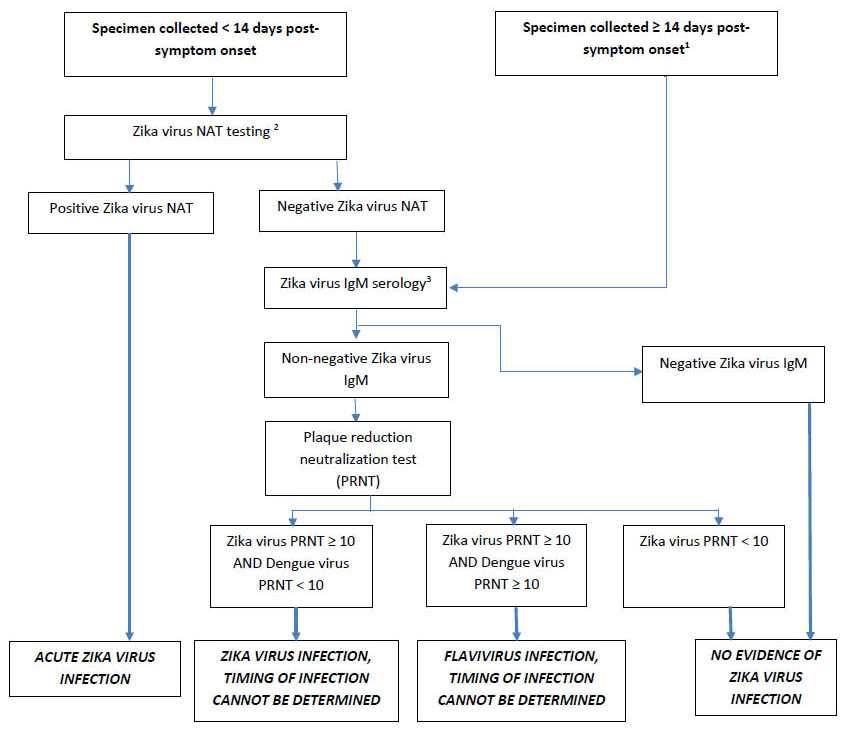
1NAT testing is not recommended for specimens obtained ≥ 14 days post-symptom onset.
2Acceptable specimens for NAT testing include serum, or patient-matched serum and urine. Repeat NAT testing of a positive result is not indicated. Dengue and chikungunya virus NAT testing should be performed for patients at risk of exposure and with clinically compatible illness.
3Dengue IgM serology should also be performed for patients at risk of exposure, and with clinically compatible illness.
Figure [Testing Recommendations for Symptomatic Non-Pregnant Individuals with Exposure to Zika Virus] depicts the order of specimen testing for specimens collected < 14 days and ≥ 14 days following Zika virus symptom onset from non-pregnant individuals. Test specimens are serum and urine, and possibly plasma, whole blood, cerebrospinal fluid (CSF), and amniotic fluid. Initially, serum, or patient-matched serum and urine specimens, collected < 14 days post-symptom onset are tested by the real-time reverse transcription polymerase chain reaction test for the Zika virus RNA nucleic acid test (NAT). Any specimen positive for Zika virus NAT is reported as an acute Zika virus infection. If results are negative for Zika virus NAT, serum samples should undergo serological testing for Zika virus IgM. If any IgM assay yields positive or equivocal results, results must be confirmed by the plaque reduction neutralization test (PRNT). Zika virus NAT is not recommended for specimens collected ≥ 14 days post-symptom onset should undergo serological testing for Zika virus IgM. If any IgM assay yields positive or equivocal results, results must be confirmed by the plaque reduction neutralization test (PRNT). Note: PRNT confirmation is not currently routinely recommended for Puerto Rico. If all IgM tests are negative, no further testing is required.
- Page last reviewed: July 24, 2017
- Page last updated: July 24, 2017
- Content source:





 ShareCompartir
ShareCompartir